What Is Plasma Cell Myeloma?/Plasma Cell Myeloma is a neoplastic plasma cell disorder that usually presents after the fifth decade of life; it is rarely described in the younger population especially under 30 years of age. In approximately 2% of plasma cell myeloma, the morphology of the neoplastic cells is highly pleomorphic, quite anaplastic, and may resemble metastatic tumor cells. While this poses a challenge for morphological interpretation during diagnosis, it has been demonstrated that bone marrow morphologic features (including diffuse sheet growth pattern, immature cell morphology, and high mitotic index) significantly correlates with high-risk disease. Moreover, there is a limited description available about the morphology of the neoplastic cells when correlating the age at presentation with the clinical outcome biological behavior; hence, the need to report and collect such cases.
Plasma cell myeloma is a more complex neoplasm than suggested by the relative uniformity of its dominant plasma cells, which represent the terminal stage of normal B-cell differentiation. Phenotypic, molecular, and cellular genetic data favor the presence of a myeloma stem cell early in hematopoietic development so that, as in chronic myelogenous leukemia (CML), a far distance exists between the primordial malignant cell that was the target of malignant transformation and the dominant clinical phenotype. Traces of pre-B, myeloid, and T cells are coexpressed with the mature B-cell phenotype, an occurrence unknown in normal B-cell differentiation. Analogous to CML, disease progression is marked by disease dedifferentiation, occasionally with the cessation of myeloma protein production and development instead of extramedullary lymphomas like features with high LDH or myelodysplasia/acute myelogenous leukemia (AML) syndromes.
Alternate/Historical Names
- Multiple myeloma
- Myelomatosis
- Medullary plasmacytoma
- Kahler’s disease
Causes Of Plasma Cell Myeloma
Different environmental, infectious, and genetic factors have been identified, which predispose to lymphoma.
- Occupational exposure – herbicides, pesticides
- Infectious organisms – These include Helicobacter pylori (MALT lymphoma), Borrelia burgdorferi, Chlamydia psittaci, Campylobacter jejuni, human T- cell lymphotropic virus (adult T- cell leukemia/lymphoma), hepatitis C ( lymphoplasmacytic lymphoma, diffuse large B-cell lymphoma and marginal zone lymphoma), human herpesvirus 8 (primary effusion lymphoma and Castleman disease). Chronic stimulation of lymphoid tissue also increases the risk of lymphoma development. Persistent infection with viruses like Epstein Barr virus and cytomegalovirus also predisposes to the development of lymphoma.[rx][rx]
- Immunodeficiency – HIV infection, transplant recipients, and those with genetic immunodeficiency disorders (severe combined immunodeficiency and common variable immunodeficiency).[rx]
- Drugs – Tumour necrosis factor-alpha inhibitors are associated in particular with T- cell lymphoma. Chronic immunosuppression in post-transplant patients (both solid organ transplant and bone marrow transplant recipients) increases the risk of lymphoma.
- Autoimmune diseases – Inflammatory bowel disease (enteropathy associated lymphoma), rheumatoid arthritis and, Sjögren’s syndrome (diffuse large B-cell lymphoma)
- Geographic location – Extranodal NK/T- cell lymphoma incidence is high in Southern Asia and some parts of Latin America.[rx][rx]
Symptoms of Plasma Cell Myeloma
- Often presents with bone pain, lytic bone lesions (thoracic vertebrae most common, also ribs, skull, pelvis, femur); spinal cord compression or peripheral neuropathy are less common presenting symptoms
- Renal failure elevated creatinine hyperuricemia (renal tubular reabsorption of light chain results in damage); hypercalcemia; hypoalbuminemia; hyperviscosity in ~7% (usually IgA or IgG3)
- Recurrent infections due to impaired humoral immunity (immunoglobulin production, often < 50% normal) including Streptococcus pneumonia, Staphylococcus aureus, E. coli
- Anemia (bone marrow infiltration often in areas of most active hematopoiesis and renal failure causing loss of erythropoietin)
- Bone marrow or extramedullary involvement or lytic bone lesions generally associated with advanced disease
- Fun fact: Bence-Jones proteins were the first tumor marker
- References: Approaches to diagnosis, assessment of disease severity and treatment of AL amyloidosis (Clin J Am Soc Nephrol 2006;1:1331), Clinicopathologic data reviewed for 15 cases to delineate the pathology of immunoglobulin M producing multiple myeloma (Am J Clin Pathol 2013;140:519), Workshop focused on salient diagnostic, clinical and genetic features of plasma cell myeloma (Am J Clin Pathol 2011;136:168)
- Asymptomatic (smoldering): more likely to progress to symptomatic myeloma than monoclonal gammopathy of uncertain significance (MGUS); both show gammopathy without symptoms
- Risk of progression 10% per year for the first 5 years
- Lower risk if no progression in the first 5 years after diagnosis (N Engl J Med 2007;356:2582)
- Nonsecretory myeloma ( < 5% of cases): SPE / IFE negative, 85% with impaired secretion and have cytoplasmic Ig by IHC
- 15% nonproducers, serum-free light chain may still be detected
- Lower incidence of renal insufficiency, hypercalcemia, and depression of normal IgG
- Plasma cell leukemia: aggressive with short survival
- > 2 x 109/L or 20% of the leukocyte count on differential are monoclonal plasma cells
- May be primary plasma cell leukemia ( < 5% of myeloma) or represent a late-stage transformation (secondary)
- Typically lack CD56, more frequent abnormal karyotype
- Bone pain and osteolytic lesions less common
- Myeloma cells have the ability to survive and grow outside of the marrow
- Typically associated with extramedullary lesions (e.g. skin, pleural effusions, lymphadenopathy, and organomegaly)
Diagnosis of Plasma Cell Myeloma
The division into these categories will guide the plan for therapy:
- Symptomatic (active) myeloma (Swerdlow: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue, Fourth Edition, 2008):
-
-
- M protein in serum or urine (usually > 30 g/L of IgG or > 25 g/L IgA or > 1 g/24 hr of urine light chain); no level included in criteria
- Clonal plasma cells in bone marrow or plasmacytoma (usually > 10%, not required)
- Related organ or tissue impairment (CRAB = hypercalcemia, renal failure/insufficiency, anemia, lytic bone lesions)
- Revised NCCN guidelines (J Natl Compr Canc Netw 2016;14:389) diagnostic criteria differ slightly from the WHO 2008 monograph with diagnostic criteria continuing to evolve:
- Clonal bone marrow plasma cells ≥ 10% or biopsy-proven bony or extramedullary plasmacytoma
- And at least one myeloma defining event:
- Calcium > 1 mg/dL above upper limit of normal or > 11 mg/dL
- Creatinine > 2 mg/dL or creatinine clearance < 40 ml/min
- Hemoglobin < 10 g/dL or > 2 g/dL below lower limit of normal
- One or more osteolytic bone lesions on the skeletal radiograph, CT or PET / CT
- Clonal bone marrow plasma cells ≥ 60%
- Involved / uninvolved light chain ratio ≥ 100
- > 1 focal lesions on MRI studies > 5 mm
- Asymptomatic (smoldering) myeloma (J Natl Compr Canc Netw 2016;14:389):
- M protein in serum at ≥ 3 g/dL when IgG or IgA
- Or
- Bence-Jones protein ≥ 500 mg/24h
- 10 – 60% clonal plasma cells in bone marrow
- And
- No related tissue damage/myeloma defining event or amyloidosis; if bone survey negative, the bone disease should be assessed with whole-body MRI or PET / CT
-
- 97% have an M protein in serum or urine, 3% are nonsecretory
- IgG (50%), IgA (20%), light chain (20%), others < 10% (IgD, IgE, IgM and biclonal)
- Serum protein electrophoresis (SPEP): serum proteins normally separate into 5 major fractions based on electric charge and size
- Albumin
- Alpha1 globulins
- Alpha2 globulins
- Beta1 and beta2 globulins
- Gamma globulins
- Gamma globulins include polyclonal antibodies and light chains, with a normal gamma zone appearing as asymmetrical smear, but there is a peak in myeloma.
- Urine protein electrophoresis (UPEP): monoclonal light chains in urine = Bence-Jones protein
- Immunofixation electrophoresis (IFE): used to characterize the M spike, by reacting with specific antisera to heavy chains IgG, IgA, IgM, IgD, IgE, and kappa and lambda light chains.
- Serum-free light chain assay (SFLCA) (Freelite): more sensitive for monitoring light chain disease and nonsecretory myeloma.
| Blood |
| Complete blood count with differential, peripheral blood smear |
| BUN, creatinine, liver enzymes, bilirubin, alkaline phosphatase, total protein, CRP |
| Tumor lysis parameters (LDH, uric acid, calcium, phosphate, potassium) |
| β2-microglobulin and albumin |
| Serum protein electrophoresis, immunofixation, serum-free light chain analysis |
| Immunophenotyping |
| Urine |
| 24-hour urine collection for electrophoresis and immunofixation |
| 24-hour urine for total protein |
| BM |
| Biopsy for histology |
| Aspirate for: |
| Morphology |
| Immunophenotyping |
| Cytogenetic analysis by FISH* focused on del(17p13), del(13q), del(1p21), ample(1q21), t(11;14), t(4;14), and t(14;16) |
| Radiographic skeletal survey, including skull, pelvis, vertebral column, and long bones |
| Additional investigations, which may be useful under certain circumstances |
| Lumbar puncture (cell counts, chemistry, cytology, immunophenotyping): suspicion of leptomeningeal involvement |
| MRI: evaluation of cord compression or painful area of the skeleton (suspicion of soft tissue plasmacytomas arising from bone) |
| CT or 18F-FDG-PET/CT: suspicion of extramedullary plasmacytomas |
| Survey for evaluation of AL amyloidosis |
| Bleeding time, APTT, PT |
| Cryoglobulins, cold agglutinins |
| Serum viscosity, fundoscopy: symptoms of hyperviscosity |
| HLA typing: in case allo-SCT is considered |
- Physical exam and health history – An exam of the body to check general signs of health, including checking for signs of disease, such as lumps or anything else that seems unusual. A history of the patient’s health habits and past illnesses and treatments will also be taken.
- Blood and urine immunoglobulin studies – A procedure in which a blood or urine sample is checked to measure the amounts of certain antibodies (immunoglobulins). For multiple myeloma, beta-2-microglobulin, M protein, free light chains, and other proteins made by the myeloma cells are measured. A higher-than-normal amount of these substances can be a sign of disease.
- Urine Tests – The proteins that myeloma cells make don’t just show up in your blood. They can appear in your urine as well, which gives doctors another way to diagnose the disease. Plus, multiple myeloma can damage your kidneys, so your doctor will want to check your pee for any sign they aren’t working properly. Some of the tests require you to collect urine over a 24-hour period, not give just one sample.
- Bone marrow aspiration and biopsy – The removal of bone marrow, blood, and a small piece of bone by inserting a hollow needle into the hipbone or breastbone. A pathologist views the bone marrow, blood, and bone under a microscope to look for abnormal cells. The following tests may be done on the sample of tissue removed during the bone marrow aspiration and biopsy:
- Cytogenetic analysis – A laboratory test in which the chromosomes of cells in a sample of bone marrow are counted and checked for any changes, such as broken, missing, rearranged, or extra chromosomes. Changes in certain chromosomes may be a sign of cancer. Cytogenetic analysis is used to help diagnose cancer, plan treatment, or find out how well treatment is working.
- FISH (fluorescence in situ hybridization) – A laboratory test used to look at and count genes or chromosomes in cells and tissues. Pieces of DNA that contain fluorescent dyes are made in the laboratory and added to a sample of a patient’s cells or tissues. When these dyed pieces of DNA attach to certain genes or areas of chromosomes in the sample, they light up when viewed under a fluorescent microscope. The FISH test is used to help diagnose cancer and help plan treatment.
- Flow cytometry – A laboratory test that measures the number of cells in a sample, the percentage of live cells in a sample, and certain characteristics of the cells, such as size, shape, and the presence of tumor (or other) markers on the cell surface. The cells from a sample of a patient’s bone marrow are stained with a fluorescent dye, placed in a fluid, and then passed one at a time through a beam of light. The test results are based on how the cells that were stained with the fluorescent dye react to the beam of light. This test is used to help diagnose and manage certain types of cancers, such as leukemia and lymphoma.
- Skeletal bone survey – In a skeletal bone survey, x-rays of all the bones in the body are taken. The x-rays are used to find areas where the bone is damaged. An x-ray is a type of energy beam that can go through the body and onto film, making a picture of areas inside the body.
- Complete blood count (CBC) with differential – A procedure in which a sample of blood is drawn and checked for the following:
- The number of red blood cells and platelets.
- The number and type of white blood cells.
- The amount of hemoglobin (the protein that carries oxygen) in the red blood cells.
- The portion of the blood sample made up of red blood cells.
- Blood chemistry studies – A procedure in which a blood sample is checked to measure the amounts of certain substances, such as calcium or albumin, released into the blood by organs and tissues in the body. An unusual (higher or lower than normal) amount of a substance can be a sign of disease.
- Twenty-four-hour urine test – A test in which urine is collected for 24 hours to measure the amounts of certain substances. An unusual (higher or lower than normal) amount of a substance can be a sign of disease in the organ or tissue that makes it. A higher than normal amount of protein may be a sign of multiple myeloma.
- MRI (magnetic resonance imaging) – A procedure that uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the body. This procedure is also called nuclear magnetic resonance imaging (NMRI). An MRI of the spine and pelvis may be used to find areas where the bone is damaged.
- PET scan (positron emission tomography scan) – A procedure to find malignant tumor cells in the body. A small amount of radioactive glucose (sugar) is injected into a vein. The PET scanner rotates around the body and makes a picture of where glucose is being used in the body. Malignant tumor cells show up brighter in the picture because they are more active and take up more glucose than normal cells do.
- CT scan (CAT scan) – A procedure that makes a series of detailed pictures of areas inside the body, such as the spine, taken from different angles. The pictures are made by a computer linked to an x-ray machine. A dye may be injected into a vein or swallowed to help the organs or tissues show up more clearly. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography.
- PET-CT scan – A procedure that combines the pictures from a positron emission tomography (PET) scan and a computed tomography (CT) scan. The PET and CT scans are done at the same time with the same machine. The combined scans give more detailed pictures of areas inside the body, such as the spine, than either scan gives by itself.
- Molecular Tests – These highly detailed looks at your bone marrow or tumor cells can identify chromosomes, genes, proteins, and other things that are unique to your cancer. The names of some of these tests are cytogenetics and fluorescent in situ hybridization (FISH). You and your doctors can use the information from these tests to decide on your treatment plan.
- Bone Marrow Biopsy – Multiple myeloma starts in bone marrow, the spongy tissue inside some bones. To test it, the doctor uses medicine to numb the area near your pelvis, then they take a sample of the liquid inside your bone marrow using a needle that goes into your pelvic bone. They also remove a sliver of bone and marrow. Doctors will check the samples to see how your cells look and whether you have too many plasma cells, a sign of multiple myeloma.
Treatment of Plasma Cell Myeloma
Conventional chemotherapy
The prognosis of pPCL after conventional chemotherapy without novel agents is poor, with median OS of ∼ 7 months.[rx–rx] There appears to be limited benefit in terms of survival for multiagent conventional chemotherapy, such as vincristine, adriamycin, and dexamethasone (VAD)-based regimens, compared with regimens containing only an alkylating agent plus a corticosteroid.[rx–rx]
Novel agents
The introduction of immunomodulatory drugs and proteasome inhibitors has significantly improved the survival of MM patients.[rx,rx] Increasing evidence suggests that these agents also improve the outcome of pPCL, but the benefit may be less pronounced compared with classic MM. A retrospective analysis performed by the Intergroup Francophone du Myélome showed that pPCL patients treated with novel agents had a survival of 15 months compared with 8 months for patients who did not receive novel agents as part of their treatment.[rx] In addition, a retrospective analysis performed by GIMEMA showed improved survival for those patients who received bortezomib and/or thalidomide at any stage of their treatment.[rx] In contrast, a SEER database analysis failed to show enhanced survival of pPCL patients in the period 1973-2004, but information on treatment changes over time were lacking in this study.[rx] There remains a limited number of prospective studies evaluating novel agents in pPCL, with several retrospective studies providing additional information on the efficacy of these drugs.
Drug therapy
Corticosteroids are steroids that have antitumor effects in multiple myeloma. Drugs such as prednisone and dexamethasone can boost your immune system, fight inflammation, and work against the myeloma cells in your body. Your doctor might have you take a steroid as part of your treatment plan. You can take these drugs in pill form, or get them as shots into a vein in your arm.
Targeted therapy
Targeted therapy is a treatment that uses drugs or other substances to identify and attack specific cancer cells. Targeted therapy may cause less harm to normal cells than chemotherapy or radiation therapy do. Several types of targeted therapy may be used to treat multiple myeloma and other plasma cell neoplasms. There are different types of targeted therapy:
- Proteasome inhibitor therapy – This treatment blocks the action of proteasomes in cancer cells. A proteasome is a protein that removes other proteins no longer needed by the cell. When the proteins are not removed from the cell, they build up and may cause the cancer cell to die. Bortezomib, carfilzomib, and ixazomib are proteasome inhibitors used in the treatment of multiple myeloma and other plasma cell neoplasms.
- Monoclonal antibody therapy – This treatment uses antibodies made in the laboratory, from a single type of immune system cell. These antibodies can identify substances on cancer cells or normal substances that may help cancer cells grow. The antibodies attach to the substances and kill the cancer cells, block their growth, or keep them from spreading. Monoclonal antibodies are given by infusion. They may be used alone or to carry drugs, toxins, or radioactive material directly to cancer cells. Daratumumab and elotuzumab are monoclonal antibodies used in the treatment of multiple myeloma and other plasma cell neoplasms. Denosumab is a monoclonal antibody used to slow bone loss and reduce bone pain in patients with multiple myeloma.
- Histone deacetylase (HDAC) inhibitor therapy – This treatment blocks enzymes needed for cell division and may stop the growth of cancer cells. Panobinostat is an HDAC inhibitor used in the treatment of multiple myeloma and other plasma cell neoplasms.
- BCL2 inhibitor therapy – This treatment blocks a protein called BCL2. Blocking this protein may help kill cancer cells and may make them more sensitive to anticancer drugs. Venetoclax is a BCL2 inhibitor being studied in the treatment of relapsed or refractory multiple myeloma.
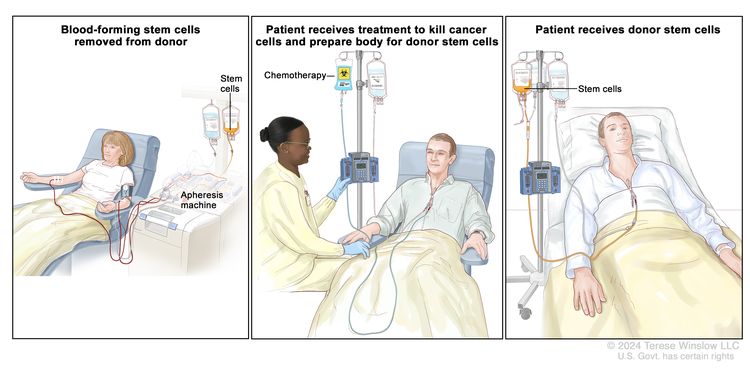
High-dose chemotherapy with stem cell transplant
High doses of chemotherapy are given to kill cancer cells. Healthy cells, including blood-forming cells, are also destroyed by cancer treatment. A stem cell transplant is a treatment to replace the blood-forming cells. Stem cells (immature blood cells) are removed from the blood or bone marrow of the patient (autologous) or a donor (allogeneic) and are frozen and stored. After the patient completes chemotherapy, the stored stem cells are thawed and given back to the patient through an infusion. These reinfused stem cells grow into (and restore) the body’s blood cells.
Immunotherapy
Immunotherapy is a treatment that uses the patient’s immune system to fight cancer. Substances made by the body or made in a laboratory are used to boost, direct, or restore the body’s natural defenses against cancer. This type of cancer treatment is also called biotherapy or biologic therapy.
- Immunomodulator therapy – Thalidomide, lenalidomide, and pomalidomide are immunomodulators used to treat multiple myeloma and other plasma cell neoplasms.
- Interferon – This treatment affects the division of cancer cells and can slow tumor growth.
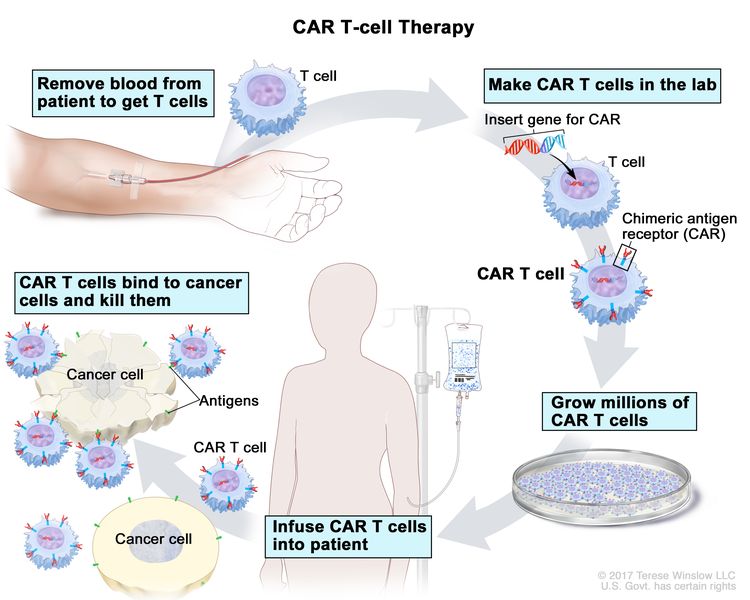
- CAR T-cell therapy – This treatment changes the patient’s T cells (a type of immune system cell) so they will attack certain proteins on the surface of cancer cells. T cells are taken from the patient and special receptors are added to their surface in the laboratory. The changed cells are called chimeric antigen receptor (CAR) T cells. The CAR T cells are grown in the laboratory and given to the patient by infusion. The CAR T cells multiply in the patient’s blood and attack cancer cells. CAR T-cell therapy is being studied in the treatment of multiple myeloma that has recurred (come back).
CAR T-cell therapy – A type of treatment in which a patient’s T cells (a type of immune cell) are changed in the laboratory so they will bind to cancer cells and kill them. Blood from a vein in the patient’s arm flows through a tube to an apheresis machine (not shown), which removes the white blood cells, including the T cells, and sends the rest of the blood back to the patient. Then, the gene for a special receptor called a chimeric antigen receptor (CAR) is inserted into the T cells in the laboratory. Millions of the CAR T cells are grown in the laboratory and then given to the patient by infusion. The CAR T cells are able to bind to an antigen on the cancer cells and kill them.
Radiation therapy
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. External radiation therapy uses a machine outside the body to send radiation toward the area of the body with cancer.
Watchful waiting
Watchful waiting is closely monitoring a patient’s condition without giving any treatment until signs or symptoms appear or change. When you have multiple myeloma, cancer cells crowd out healthy blood cells in your body. Instead of making normal proteins called antibodies, they make particles called M proteins. (The M stands for monoclonal.) To diagnose you, your doctor will take a small amount of blood from a vein in your arm. A lab then checks it for M proteins and another substance — beta-2 microglobulin — that are signs you have multiple myeloma.
Supportive care is given to lessen the problems caused by the disease or its treatment.
This therapy controls problems or side effects caused by the disease or its treatment, and improves quality of life. Supportive care is given to treat problems caused by multiple myeloma and other plasma cell neoplasms.
Supportive care may include the following:
- Plasmapheresis – If the blood becomes thick with extra antibody proteins and interferes with circulation, plasmapheresis is done to remove extra plasma and antibody proteins from the blood. In this procedure blood is removed from the patient and sent through a machine that separates the plasma (the liquid part of the blood) from the blood cells. The patient’s plasma contains the unneeded antibodies and is not returned to the patient. The normal blood cells are returned to the bloodstream along with donated plasma or a plasma replacement. Plasmapheresis does not keep new antibodies from forming.
- High-dose chemotherapy with stem cell transplant – If amyloidosis occurs, treatment may include high-dose chemotherapy followed by stem cell transplant using the patient’s own stem cells.
- Immunotherapy – Immunotherapy with thalidomide, lenalidomide, or pomalidomide is given to treat amyloidosis.
- Targeted therapy – Targeted therapy with proteasome inhibitors is given to decrease how much immunoglobulin M is in the blood and treat amyloidosis. Targeted therapy with a monoclonal antibody is given to slow bone loss and reduce bone pain.
- Radiation therapy – Radiation therapy is given for bone lesions of the spine.
- Chemotherapy – Chemotherapy is given to reduce back pain from osteoporosis or compression fractures of the spine.
- Bisphosphonate therapy – Bisphosphonate therapy is given to slow bone loss and reduce bone pain. See the following PDQ summaries for more information on bisphosphonates and problems related to their use:
- Cancer Pain
- Oral Complications of Chemotherapy and Head/Neck Radiation
Bortezomib
In MM, proteasome inhibition with bortezomib has shown the ability to (partly) overcome the prognostic adverse impact of high-risk cytogenetic aberrations such as t(4;14), t(14;16), t(14;20), del(1p), and del(17p) [rx–rx]. Adverse cytogenetic findings are common in PCL, and bortezomib might be particularly well suited to include in the treatment. The first major study showing promising results was conducted by the GIMEMA group in 2012 [rx]. In 2016, a French prospective phase 2 study tested the efficacy of bortezomib in combination with dexamethasone, and either doxorubicin or cyclophosphamide followed by high-dose melphalan and ASCT. This study showed a high overall response rate (69%) and OS of 36.3 months [rx]. Most retrospective studies have supported an important role of bortezomib in PCL treatment [rx, rx, rx]; only an Israeli study did not report improved survival after treatment with bortezomib or carfilzomib [rx].
Thalidomide
The efficacy of single-agent thalidomide is limited in pPCL compared with the activity of this agent in MM.[rx,rx] Although some reports with small numbers of patients showed that thalidomide may result in durable responses in sPCL or pPCL, its decreased activity in extramedullary MM makes its use less attractive.[rx,rx] Conversely, addition of thalidomide to dexamethasone, conventional chemotherapy, or bortezomib may result in enhanced activity in pPCL.[rx,rx,rx]
Lenalidomide
Lenalidomide is less toxic and more potent than thalidomide. The combination of lenalidomide with dexamethasone has been effective in newly diagnosed pPCL.[rx] In a prospective phase 2 study, 23 newly diagnosed pPCL patients were treated with lenalidomide and dexamethasone: 14 patients completed the initial 4 planned cycles and partial response (PR) was achieved in 61%, with ≥ VGPR in 35%. Five patients underwent auto-SCT and 1 received tandem auto-SCT/allogeneic SCT (allo-SCT) after lenalidomide plus dexamethasone treatment. After a median follow-up of 15 months, OS and PFS were 63% and 52%, respectively.[rx] Lenalidomide-based therapies also appear promising in the setting of relapsed/refractory disease, especially in combination with bortezomib.[rx,rx,rx]
Combinations of novel agents
In pPCL, the efficacy of combinations of novel agents, such as lenalidomide, bortezomib, and dexamethasone (RVD),[rx] bortezomib, thalidomide, and dexamethasone (VTD),rx,rx,rx] or melrxzphalan, prednisone, bortezomib, and thalidomide (VMPT),[rx] appears very promising. Studies describing these regimens invoke only a small numbers of patients but are based on the biologic and clinical features seen.
Auto-SCT
McElwain and Powles were the first to describe the efficacy of high-dose melphalan in a pPCL patient, who survived more than 30 months after melphalan 140 mg/m2.[rx] Since then, other case reports and small case series suggest that high-dose therapy with hematopoietic stem cell support improves OS.[x,rx,rx,rx,rx]
The largest retrospective analysis to date was performed by the European Group for Blood and Marrow Transplantation,[rx] who compared 272 pPCL patients with 20 844 MM patients undergoing auto-SCT between 1980 and 2006.[rx] Although CR rates before and after autologous SCT were higher in pPCL patients, median PFS (14.3 vs 27.4 months) and OS (25.7 vs 62.3 months) were significantly longer in MM patients. Conversion to CR after auto-SCT was associated with improved PFS and OS.[rx] Treatment-related mortality (TRM) was higher in the pPCL group. Importantly, this study lacked information regarding type of induction regimen, which is probably critical based on current data.
AlloSCT Compared to ASCT
So far, published data seem to favor ASCT compared to alloSCT. However, no prospective studies have made a direct comparison. AlloSCT has mostly been used in combination with ASCT, using ASCT to deepen response before alloSCT administered with reduced-intensity conditioning [rx, rx, rx]. The poorer reported results with alloSCT could partly be caused by selection bias where patients with particularly aggressive disease behavior have been allotransplanted. Another reason for poorer outcomes after alloSCT is high treatment-related mortality. Historically, this has been high, but within the last decennium, it has decreased. The potential role of alloSCT has not been finally settled. Graft-versus-tumor effect after donor lymphocyte infusion has been documented in MM [rx, rx], but could be less in PCL posts maintenance could improve disease control until the GVL effect is mature, and moreover, lenalidomide, other IMId, or immunotherapy could enhance the GVL effect [rx]. Ongoing trials, including the European primary PCL study (EudraCT number 2013-005157-75), will contribute to clarify the role of alloSCT in PCL.
Proteasome inhibitors
The ubiquitin‐proteasome pathway plays an important role in tumorigenesis and cell proliferation by producing substrates required for synthesis and protein modification. Bortezomib is a ubiquitin‐proteasome inhibitor with pro‐apoptotic properties that is approved for the treatment of MM.[rx] Few previous retrospective analysis have shown bortezomib‐based regimens showed efficacy with OS of about 18 to 28 months in patients with plasma cell leukemia.[rx, rx, rx In 2016, a multicenter phase 2 prospective trial that enrolled 40 patients with pPCL aged 70 years or less for four alternating cycles of bortezomib, dexamethasone plus doxorubicin or cyclophosphamide.[rx] Patients then received high‐dose melphalan followed by AuSCT.[rx] Median PFS was 15.1 months, and the OS was 36.3 months. The study concluded that patients with bortezomib combination therapy followed by transplantation induced high response rates and improved PFS.[rx]
Synergism between IMiDs and proteasome inhibitors can be utilized in the treatment of patients refractory to lenalidomide. This synergism can be explained by a novel mechanism of action of lenalidomide which involves increased protein ubiquitination.[rx]
Bcl‐2 inhibitors: Venetoclax
The intrinsic apoptosis pathway is regulated by competing anti‐apoptotic (eg, Bcl‐2, Mcl‐1) and pro‐apoptotic proteins (eg, Bax, Bak, Bim). Cell death is induced when cellular injury causes the release of pro‐apoptotic proteins which are normally sequestered by anti‐apoptotic proteins. These pro‐apoptotic proteins translocate to the mitochondrial outer membrane and initiate a cascade that leads to increased mitochondria permeability and apoptosis.[rx] Bcl‐2 family proteins are highly associated with the survival of clonal plasma cell malignancies, whose targeted inhibition has been used in the setting of refractory disease with efficacy in those with t (11;14) abnormality.[rx] Venetoclax is a selective oral Bcl‐2‐specific BH3 mimetic that is FDA approved for the treatment of chronic lymphocytic leukemia (CLL).[rx] It has been shown in a Phase I study in those with relapsed or refractory multiple myeloma with t (11;14) abnormality to have an overall response rate (ORR) of 21% and very good partial response or better in 15%.[rx] Mcl‐1, another Bcl‐2 family protein, has been associated with resistance to Bcl‐2 inhibition when overexpressed in clonal plasma cell malignancies. Theoretically, inhibition of Mcl‐1 with bortezomib in combination with venetoclax is synergistic. A combination regimen of daratumumab‐venetoclax‐bortezomib‐dexamethasone was reported to lead to disease remission in a patient refractory to proteasome inhibitors after three cycles of therapy with repeat bone marrow biopsy showing no morphological or immunophenotypical evidence of clonal plasma cells.[rx] Dexamethasone can upregulate the expression of pro‐apoptotic molecule BIM and has been shown to have high response rates when used in combination with venetoclax and bortezomib irrespective of t (11;14) status.[rx]
Anti‐CD38: Daratumumab
Natural killer (NK) cell‐based immunotherapy is a therapeutic approach to refractory MM. Daratumumab, an engineered monoclonal antibody (IgG1k), binds to CD38 surface antigen that is overexpressed in MM cells. This leads to activation of NK antibody‐dependent cellular cytotoxicity (ADCC) activity.[rx] However, their antitumor effects seem dependent on CD38 expression since ADCC activity was largely absent in CD38 low or negative cells.[rx] Daratumumab can exert toxic effect on healthy cells, leading to decreased number of NK cells. Termed fratricide, a phenomenon whereby binding of the mAb against CD38+ NK cells leads to ADCC activation against other CD38+ NK cells bound to daratumumab.[rx] It is approved by the Food and Drug Administration (FDA) initially in 2015 as a second‐line agent in the treatment of MM in patients who have received at least three prior therapies. The following year, the FDA further expanded the use of daratumumab in combination with lenalidomide or bortezomib and dexamethasone in those who have received at least one prior therapy. Beginning in May 2018, it can be used in combination with bortezomib, melphalan, and prednisone for treatment‐naive MM who are transplant‐ineligible. There are currently no published trials that demonstrate its efficacy in pPCL. There is currently one case report that showed a rapid and deep response in a pPCL with t(11;14).[rx]
Treatment of sPCL
For sPCL, studies are extremely limited, and patients are often heavily pretreated. A recent study indicated improved prognosis by treatment with bortezomib-containing regimens. The study reported the most important factor to be a high-quality first response to treatment [rx]. A recent, small study further investigated the treatment of sPCL with bortezomib and lenalidomide-containing regimen achieving PFS of more than 27 months in 2/9 pts. [rx]. As in MM, treatments with thalidomide and lenalidomide are likely to have some effect on sPCL, but patients will often already have received these treatments [rx]. ASCT has been used for sPCL. The survival was still poor, but a few patients achieved remission for more than 1 year [rx].
New and Upcoming Treatments and Studies
- Venetoclax – is a BCL-2 inhibitor that has demonstrated remarkable efficacy in MM, CLL, and other hematological diseases harboring the (11;14) translocation [rx]. As noted earlier, this particular translocation is common in PCL. In a recent case report, venetoclax was used in combination with daratumumab, dexamethasone, and bortezomib in a t(11;14) refractory PCL patient resulting in a rapid and deep response already after the first treatment cycle [rx].
- Pomalidomide – is a third-generation IMID that has shown good response and survival benefit in refractory MM [rx, rx]. In a case report, a PCL patient with CNS relapse after allogeneic SCT was successfully treated with cerebral radiation and intrathecal chemotherapy followed by pomalidomide and dexamethasone maintenance. At the time of reporting, the patient was still in remission after 18 months of follow-up [rx]. In another case report, a patient with sPCL achieved normalization of hematological values and a significant decrease in M-component after a 4-month treatment combining low-dose dexamethasone and pomalidomide [rx].
- Ixazomib – is a second-generation PI used in combination with lenalidomide and dexamethasone in relapsed or refractory MM [rx, rx]. Ixazomib is currently being investigated in a phase 1b study as maintenance treatment after alloSCT in relapsed high-risk MM included patients with sPCL and PCL [rx]. An ongoing phase II study from the Mayo Clinic investigates the efficacy of combining ixazomib, pomalidomide, and dexamethasone for sPCL or previously treated MM [rx].
- Carfilzomib – another second-generation PI, combined with lenalidomide and dexamethasone is currently being tested as induction treatment of PCL in a European multi-center study (EudraCT number 2013-005157-75). Responding transplant eligible patients are subsequently treated with ASCT followed by allo-SCT, and hereafter maintained with low-dose lenalidomide to increase GVL effect.
- Daratumumab – is an anti-CD38 antibody which, in several studies, has shown impressive efficacy in relapsed, refractory MM. Daratumumab and other anti-CD38 antibodies will for sure be of high interest to study in PCL and sPCL [rx]. The use of anti-CD45 antibodies is currently being investigated for high-risk myeloma, [rx], and other antibodies-targeting CD75s are currently being investigated for their ability to bind MM and PCL cells [rx].
- BRAF/MEK inhibitors – are newly developed compounds that are used successfully for targeted treatment of malignant melanoma [rx] and MM [rx]. BRAF pathway mutations are seen in about 5–6% of MM patients, whereas BRAF pathway mutations are less frequently observed in PCL compared to sPCL and MM. The treatment principle of combining BRAF and MEK inhibitors will be an interesting option in the treatment of BRAF pathway-mutated PCL or sPCL patients [rx].
- CAR-T therapy – is an exciting new technique using genetically engineered autologous T cells that are programmed to bind specific antigens on target cells. Encouraging results have been found in lymphomas and leukemia and also in MM [69]. Data in the PCL setting is pending.
- Peptide vaccination – studies have so far not fulfilled their promises in MM. However, studies are ongoing, also including PCL patients [rx].
References