Neurotransmitters and Receptors/Neurotransmitters are chemical messengers that transmit a signal from a neuron across the synapse to a target cell, which can be a different neuron, muscle cell, or gland cell. Neurotransmitters are chemical substances made by the neuron specifically to transmit a message.[rx]
Neurotransmitters are released from synaptic vesicles in synapses into the synaptic cleft, where they are received by neurotransmitter receptors on the target cell. Many neurotransmitters are synthesized from simple and plentiful precursors such as amino acids, which are readily available and only require a small number of biosynthetic steps for conversion. Neurotransmitters are essential to the function of complex neural systems. The exact number of unique neurotransmitters in humans is unknown, but more than 500 have been identified.[rx][rx][rx]
Neurotransmitter, also called chemical transmitter or chemical messenger, any of a group of chemical agents released by neurons (nerve cells) to stimulate neighboring neurons or muscle or gland cells, thus allowing impulses to be passed from one cell to the next throughout the nervous system. The following is an overview of neurotransmitter action and types; for more information, see the nervous system.
Cholinergic Neurons and Receptors
Acetylcholine is a neurotransmitter in the central and peripheral nervous systems that affect plasticity, arousal, and reward.
Key Points
The neurotransmitter acetylcholine (ACh) is the only neurotransmitter used in the motor division of the somatic nervous system and the principal neurotransmitter at autonomic ganglia.
In the CNS, the neurons that release and respond to ACh comprise the cholinergic system, which causes anti-excitatory effects.
ACh plays a role in synaptic plasticity, including learning and short-term memory.
ACh may bind either muscarinic or nicotinic receptors.
ACh is synthesized in cholinergic neurons (such as those in the nucleus basalis of Meynert) from choline and acetyl-CoA using an enzyme called choline acetyltransferase.
Key Terms
choline acetyltransferase: Abbreviated as ChAT, this is an enzyme that is synthesized within the body of a neuron. It is then transferred to the nerve terminal via axoplasmic flow. The role of choline acetyltransferase is to join Acetyl-CoA to choline, resulting in the formation of the neurotransmitter acetylcholine.
autonomic ganglia: Clusters of neuronal cell bodies and their dendrites that are a junction between the autonomic nerves originating from the central nervous system and the autonomic nerves innervating their target organs in the periphery.
nicotinic receptors: Also called nAChRs, these are cholinergic receptors that form ligand-gated ion channels in the plasma membranes of certain neurons and on the postsynaptic side of the neuromuscular junction.
Acetylcholine
Acetylcholine (ACh) is an organic, polyatomic ion that acts as a neurotransmitter in both the peripheral nervous system (PNS) and central nervous system (CNS) in many organisms, including humans. Acetylcholine is one of many neurotransmitters in the autonomic nervous system (ANS) and the only neurotransmitter used in the motor division of the somatic nervous system (sensory neurons use glutamate and various peptides at their synapses ).
Acetylcholine is also the principal neurotransmitter in all autonomic ganglia. In cardiac tissue, acetylcholine neurotransmission has an inhibitory effect, which lowers heart rate. However, acetylcholine also behaves as an excitatory neurotransmitter at neuromuscular junctions in skeletal muscle.

Acetylcholine: The chemical structure of acetylcholine is depicted.
Acetylcholine was first identified in 1914 by Henry Hallett Dale for its actions on heart tissue. It was confirmed as a neurotransmitter by Otto Loewi, who initially gave it the name Vagusstoff because it was released from the vagus nerve. They jointly received the 1936 Nobel Prize in physiology or medicine for their work. Acetylcholine was also the first neurotransmitter to be identified.
Functions
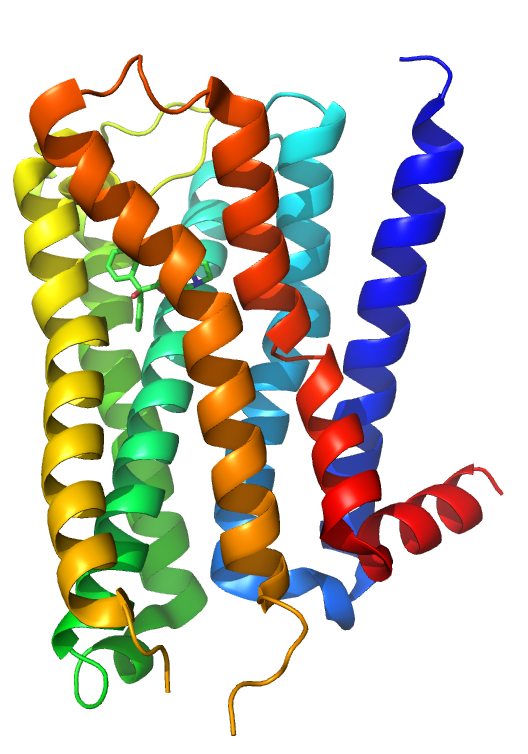
Muscarinic acetylcholine receptor M2: This human M2 muscarinic acetylcholine receptor is bound to an antagonist (ACh).
Acetylcholine has functions both in the peripheral nervous system (PNS) and in the central nervous system (CNS) as a neuromodulator. In the peripheral nervous system, acetylcholine activates muscles and is a major neurotransmitter in the autonomic nervous system. In the central nervous system, acetylcholine and its associated neurons form the cholinergic system.
When acetylcholine binds to acetylcholine receptors on skeletal muscle fibers, it opens ligand-gated sodium channels in the cell membrane. Sodium ions then enter the muscle cell, initiating a sequence of steps that finally produce muscle contraction. Although acetylcholine induces the contraction of skeletal muscle, it acts via a different type of receptor to inhibit the contraction of cardiac muscle fibers.
In the autonomic nervous system, acetylcholine is released in the following sites: all pre-and post-ganglionic parasympathetic neurons, all pre-ganglionic sympathetic neurons, some post-ganglionic sympathetic fibers, and in the pseudo motor neurons to sweat glands.
In the central nervous system, ACh has a variety of effects as a neuromodulator for plasticity, arousal, and reward. ACh has an important role in the enhancement of sensory perceptions when we wake up and in sustaining attention.
Damage to the cholinergic (acetylcholine-producing) system in the brain has been shown to be plausibly associated with the memory deficits associated with Alzheimer’s disease. ACh has also been shown to promote REM sleep.
In the cerebral cortex, tonic ACh inhibits layer 4 neurons, the main targets of thalamocortical inputs while exciting pyramidal cells in layers 2/3 and 5. This filters out weak sensory inputs in layer 4 and amplifies inputs that reach the layers 2/3 and layer 5 excitatory microcircuits.
As a result, these layer-specific effects of ACh might function to improve the signal-to-noise ratio of cortical processing. At the same time, acetylcholine acts through nicotinic receptors to excite certain groups of inhibitory interneurons in the cortex that further dampen cortical activity.
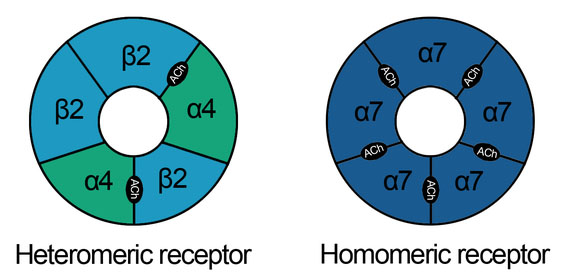
Nicotinic acetylcholine receptors: These schematics describe the heteromeric and homomeric nature of nAChRs. The heteromeric receptors found in the central nervous system are made up of 2 α and 3 β subunits with the binding site at the interface of α and adjacent subunits. Homomeric receptors contain 5 identical subunits and have 5 binding sites located at the interfaces between adjacent subunits.
One well-supported function of ACh in the cortex is increased responsiveness to sensory stimuli, a form of attention. Phasic increases of ACh during visual, auditory, and somatosensory stimulus presentations have been found to increase the firing rate of neurons in the corresponding primary sensory cortices.
When cholinergic neurons in the basal forebrain are lesioned, animals’ ability to detect visual signals was robustly and persistently impaired. In that same study, an animals’ ability to correctly reject non-target trials was not impaired, further supporting the interpretation that phasic ACh facilitates responsiveness to stimuli.
ACh has been implicated in reporting expected uncertainty in the environment, based both on the suggested functions listed above and results recorded while subjects perform a behavioral cuing task. Reaction time differences between correctly cued trials and incorrectly cued trials, called the cue validity, was found to vary inversely with ACh levels in primates with pharmacologically and surgically altered levels of ACh. The result was also found in Alzheimer’s disease patients and smokers after nicotine (an ACh agonist) consumption.
Creation of ACh
Acetylcholine is synthesized in certain neurons by the enzyme choline acetyltransferase from the compounds choline and acetyl-CoA. Cholinergic neurons are capable of producing ACh.
An example of a central cholinergic area is the nucleus basalis of Meynert in the basal forebrain. The enzyme acetylcholinesterase converts acetylcholine into the inactive metabolites choline and acetate. This enzyme is abundant in the synaptic cleft, and its role in rapidly clearing free acetylcholine from the synapse is essential for proper muscle function.
Certain neurotoxins work by inhibiting acetylcholinesterase, leading to excess acetylcholine at the neuromuscular junction. This results in paralysis of the muscles needed for breathing and stops the beating of the heart.
Adrenergic Neurons and Receptors
Adrenergic receptors are molecules that bind catecholamines. Their activation leads to overall stimulatory and sympathomimetic responses.
Key Points
Adrenergic receptors consist of two main groups, α and β, multiple subgroups (α1, α2, β1, β2, β3), and several subtypes of the α2 subgroup (α2A, α2B, α2C).
Epinephrine binds both α and β adrenergic receptors to cause vasoconstriction and vasodilation.
When activated, the α1 receptor triggers smooth muscle contraction in blood vessels in the skin, gastrointestinal tract, kidney, and brain, among other areas.
When activated, the α2 receptor triggers inhibition of insulin and the induction of glucagon release in the pancreas, contraction of GI tract sphincters, and increased thrombocyte aggregation.
When activated, the α2 receptor triggers inhibition of insulin and induction of glucagon release in the pancreas, contraction of GI tract sphincters, and increased thrombocyte aggregation.
Key Terms
adrenoreceptor: These are a class of G protein-coupled receptors that are targets of the catecholamines, especially norepinephrine (noradrenaline) and epinephrine (adrenaline). Many cells possess these receptors, and the binding of a catecholamine to the receptor will generally stimulate the sympathetic nervous system.
G protein-coupled receptors: These comprise a large protein family of transmembrane receptors that sense molecules outside the cell and activate inside signal transduction pathways and, ultimately, cellular responses. Any adrenergic effects on cells are generally mediated by G protein-coupled receptors.
adrenergic receptor: Any of several sites in the surface membranes of cells innervated by adrenergic neurons.
The adrenergic receptors (or adrenoceptors) are a class of metabotropic G protein-coupled receptors that are targets of the catecholamines, especially norepinephrine or noradrenaline, and epinephrine ( adrenaline ). Although dopamine is a catecholamine, its receptors are in a different category.
Many cells possess these receptors, and the binding of an agonist will generally cause a sympathetic (or sympathomimetic) response (e.g., the fight-or-flight response). For instance, the heart rate will increase, pupils will dilate, energy will be mobilized, and blood flow will be diverted from non-essential organs to skeletal muscle.

Adrenaline (epinephrine): The 2D structure of adrenaline (epinephrine) is illustrated.

Noradrenaline (norepinephrine): The 2D structure of noradrenaline (norepinephrine) is illustrated here.
There are two main groups of adrenergic receptors, α, and β, with several subtypes. α receptors have the subtypes α1 (a Gq coupled receptor) and α2 (a Gi-coupled receptor). Phenylephrine is a selective agonist of the α receptor.
β-receptors have the subtypes β1, β2, and β3. All three are linked to Gs proteins (although β2 also couples to Gi), which in turn are linked to adenylate cyclase. Agonist binding thus causes a rise in the intracellular concentration of the second messenger cAMP. Downstream effectors of cAMP include the cAMP-dependent protein, kinase (PKA), which mediates some of the intracellular events following hormone binding. Isoprenaline is a nonselective agonist.
Adrenaline or noradrenaline is a receptor-ligand to α1, α2, or β-adrenergic receptors (the pathway is shown in the following diagram).
- α1 couples to Gq, which results in increased intracellular Ca2+ that results in smooth muscle contraction.
- α2, on the other hand, couples to Gi, which causes a decrease of cAMP activity, that results in smooth muscle contraction.
- β receptors couple to Gs, and increases intracellular cAMP activity, resulting in heart muscle contraction, smooth muscle relaxation, and glycogenolysis.
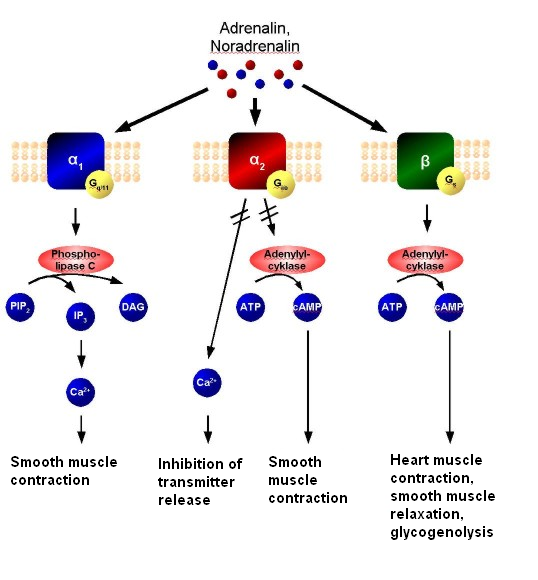
Adrenergic signal transduction: This schematic shows the mechanism of adrenergic receptors. Adrenaline and noradrenaline are ligands to α1, α2, or β-adrenergic receptors. α1-receptors couple to Gq, resulting in increased intracellular Ca2+ and causing smooth muscle contraction. α2 receptors couple to Gi, causing a decrease in cAMP activity and resulting in smooth muscle contraction. β-receptors couple to Gs, increasing intracellular cAMP activity and resulting in heart muscle contraction, smooth muscle relaxation, and glycogenolysis.
Adrenaline (epinephrine) reacts with both α- and β-adrenoceptors, causing vasoconstriction and vasodilation, respectively. Although α receptors are less sensitive to epinephrine, when activated, they override the vasodilation mediated by β-adrenoceptors. The result is that high levels of circulating epinephrine cause vasoconstriction. At lower levels of circulating epinephrine, β-adrenoceptor stimulation dominates, producing overall vasodilation.
Smooth muscle behavior is variable depending on anatomical location. One important note is the differential effects of increased cAMP in smooth muscle compared to cardiac muscle. Increased cAMP will promote relaxation in smooth muscle while promoting increased contractility and pulse rate in cardiac muscle.
α-receptors have several functions in common, but also individual effects. Common (or still unspecified) effects include: vasoconstriction of cardiac arteries (coronary artery), vasoconstriction of veins, and decreased motility of smooth muscle in the gastrointestinal tract.
α1-adrenergic receptors are members of the G protein-coupled receptor superfamily. On activation, a heterotrimeric G protein, Gq, activates phospholipase C (PLC).
The PLC cleaves phosphatidylinositol 4,5-bisphosphate (PIP2), which in turn causes an increase in inositol triphosphate (IP3) and diacylglycerol (DAG). The former interacts with calcium channels of the endoplasmic and sarcoplasmic reticulum, thus changing the calcium content in a cell. This triggers all other effects.
Specific actions of the α1-receptor mainly involve smooth muscle contraction. It causes vasoconstriction in many blood vessels, including those of the skin, gastrointestinal system, kidney (renal artery), and brain. Other areas of smooth muscle contraction are:
- Ureter.
- Vas deferens.
- Hair (arrector pili muscles).
- Uterus (when pregnant).
- Urethral sphincter.
- Bronchioles (although minor to the relaxing effect of β2 receptor on bronchioles).
- Blood vessels of the ciliary body (stimulation causes mydriasis).
Further effects include glycogenolysis and gluconeogenesis from adipose tissue and the liver, as well as secretion from sweat glands, and Na+ reabsorption from the kidney. Antagonists may be used in hypertension.
There are 3 highly homologous subtypes of α2 receptors: α2A, α2Β, and α2C.
α2-Receptor Effects
- Inhibition of insulin release in the pancreas.
- Induction of glucagon release from the pancreas.
- Contraction of sphincters of the gastrointestinal tract.
- Negative feedback in the neuronal synapses—presynaptic inhibition of noradrenaline release in CNS.
β1-Receptor Effects
- Increases cardiac output, by raising heart rate (positive chronotropic effect), increasing impulse conduction (positive dromotropic effect), and increasing contraction (positive inotropic effect), thus increasing the volume expelled with each beat (increased ejection fraction).
- Increases renin secretion from the juxtaglomerular cells of the kidney.
- Increases ghrelin secretion from the stomach.
β2-Receptor Effects
- Smooths muscle relaxation, e.g., in bronchi and the GI tract (decreased motility).
- Lipolysis in adipose tissue.
- Anabolism in skeletal muscle.
- Relaxes a non-pregnant uterus.
- Dilates arteries to skeletal muscle.
- Glycogenolysis and gluconeogenesis.
- Stimulates insulin secretion.
- Contracts the sphincters of the GI tract.
- Thickens secretions from the salivary glands.
- Inhibits histamine release from mast cells.
- Increases renin secretion from the kidney.
- Relaxation of bronchioles (salbutamol, a beta-2 agonist, relieves bronchiole constriction).
Agonists, Antagonists, and Drugs
Drugs affecting cholinergic neurotransmission may block, hinder, or mimic the action of acetylcholine and alter post-synaptic transmission.
Key Points
Acetylcholine receptor agonists and antagonists have either direct effects on the receptors or act indirectly by affecting the enzyme acetylcholinesterase.
Agents targeting ACh receptors may target either the nicotinic or muscarinic receptors for ACh.
Atropine, an antagonist for muscarinic ACh receptors, lowers the parasympathetic activity of muscles and glands in the parasympathetic nervous system.
Neostigmine is an indirect ACh receptor agonist that inhibits acetylcholinesterase, preventing the breakdown of acetylcholine. It is used in the treatment of myasthenia gravis and to reverse the effects of neuromuscular blockers used for anesthesia.
Phenylephrine, marketed as a substitute for Sudafed for decongestant purposes, is an α1- adrenergic receptor agonist.
Beta-blockers, as their name suggests, block the action of epinephrine and norepinephrine on β-adrenergic receptors and are used for the management of cardiac arrhythmias, cardio-protection after a heart attack, and hypertension.
Key Terms
acetylcholinesterase: An enzyme that catalyzes the breakdown of the neurotransmitter acetylcholine.
beta-blockers: Also called beta-adrenergic blocking agents, beta-adrenergic antagonists, beta-adrenoreceptor antagonists, or beta antagonists, these are a class of drugs used for various indications. As beta-adrenergic receptor antagonists, they diminish the effects of epinephrine (adrenaline) and other stress hormones.
atropine: An alkaloid extracted from the plant deadly nightshade (Atropa belladonna) and other sources. It is used as a drug in medicine for its paralytic effects (e.g., in surgery to relax muscles, in dentistry to dry the mouth, in ophthalmology to dilate the pupils), though overdoses are fatal.
Blocking, hindering, or mimicking the action of acetylcholine has many uses in medicine. Drugs that act on the acetylcholine system are either agonists to the receptors that stimulate the system, or antagonists that inhibit it.
Acetylcholine receptor agonists and antagonists can have a direct effect on the receptors or exert their effects indirectly. For example, by affecting the enzyme acetylcholinesterase the receptor-ligand is degraded. Agonists increase the level of receptor activation, antagonists reduce it.
Acetylcholine in the ANS
The vagus (parasympathetic) nerves that innervate the heart release acetylcholine (ACh) as their primary neurotransmitter. ACh binds to muscarinic receptors (M2) that are found principally on cells comprising the sinoatrial (SA) and atrioventricular (AV) nodes.
Muscarinic receptors are coupled to the Gi-protein; therefore, vagal activation decreases cAMP. Gi-protein activation also leads to the activation of KACh channels that increase potassium efflux and hyperpolarizes the cells.
Increases in vagal activity to the SA node decrease the firing rate of the pacemaker cells by decreasing the slope of the pacemaker potential and decreasing heart rate. By hyperpolarizing the cells, vagal activation increases the cell’s threshold for firing, which contributes to the reduction of the firing rate.
Similar electrophysiological effects also occur at the atrioventricular AV node. However, in this tissue, these changes are manifested as a reduction in impulse conduction velocity through the AV node. In the resting state, there is a large degree of vagal tone on the heart, which is responsible for low, resting heart rates.
There is also some vagal innervation of the atrial muscle, and to a much lesser extent, the ventricular muscle. Vagus activation, therefore, results in modest reductions in atrial contractility (inotropy) and even smaller decreases in ventricular contractility.
Muscarinic Antagonists

Atropine: The 2D chemical structure of atropine is illustrated here.
Muscarinic receptor antagonists bind to muscarinic receptors, thereby preventing ACh from binding to and activating the receptor. By blocking the actions of ACh, muscarinic receptor antagonists very effectively block the effects of vagal nerve activity on the heart. By doing so, they increase heart rate and conduction velocity.
Atropine is a naturally occurring tropane alkaloid extracted from deadly nightshade (Atropa belladonna), Jimson weed (Datura stramonium), mandrake (Mandragora officinarum), and other plants of the family Solanaceae. Atropine’s pharmacological effects are due to its ability to bind to muscarinic acetylcholine receptors. It is an anti-muscarinic agent.
Working as a nonselective muscarinic acetylcholinergic antagonist, atropine increases firing of the sinoatrial node (SA) and conduction through the atrioventricular node (AV) of the heart, opposes the actions of the vagus nerve, blocks acetylcholine receptor sites, and decreases bronchial secretions. In overdoses, atropine is poisonous.
Nicotinic Agonists
A nicotinic agonist is a drug that mimics, in one way or another, the action of acetylcholine (ACh) at nicotinic acetylcholine receptors (nAChRs). Nicotinic acetylcholine receptors are receptors found in the central nervous system, the peripheral nervous system, and skeletal muscles.
They are ligand-gated ion channels with binding sites for acetylcholine as well as other agonists. When agonists bind to a receptor it stabilizes the open state of the ion channel allowing an influx of cations.

Nicotinic acetylcholine receptors: NAchR are cholinergic receptors that form ligand-gated ion channels in the plasma membranes of certain neurons and on the postsynaptic side of the neuromuscular junction.
The development of nicotinic acetylcholine receptor agonists began in the early nineties after the discovery of nicotine’s positive effects on animal memory. Nicotinic antagonists are mainly used for peripheral muscle paralysis in surgery, the classical agent of this type being tubocurarine, but some centrally acting compounds such as bupropion, mecamylamine, and 18-methoxycoronaridine block nicotinic acetylcholine receptors in the brain and have been proposed for treating drug addiction.
The nicotinic acetylcholine receptor agonists are gaining increasing attention as drug candidates for multiple central nervous system disorders such as Alzheimer’s disease, schizophrenia, attention-deficit hyperactivity disorder (ADHD), and nicotine addiction. In 2009 there were at least five drugs on the market that affect the nicotinic acetylcholine receptors.
Most indirect-acting ACh receptor agonists work by inhibiting the enzyme acetylcholinesterase. The resulting accumulation of acetylcholine causes continuous stimulation of the muscles, glands, and central nervous system.
They are examples of enzyme inhibitors, and increase the action of acetylcholine by delaying degradation; some have been used as nerve agents (sarin and VX nerve gas) or pesticides (organophosphates and carbamates). In clinical use, they are administered to reverse the action of muscle relaxants, to treat myasthenia gravis, and to treat symptoms of Alzheimer’s disease (rivastigmine increases cholinergic activity in the brain).
Beta Receptor Antagonists
Beta-blockers (sometimes written as β-blockers) or beta-adrenergic blocking agents, beta-adrenergic antagonists, beta-adrenoreceptor antagonists, or beta antagonists, are a class of drugs used for various indications. They are particularly used for the management of cardiac arrhythmias, cardiac protection after myocardial infarction (heart attack), and hypertension.
As beta-adrenergic receptor antagonists, they diminish the effects of epinephrine (adrenaline) and other stress hormones. Beta-blockers block the action of endogenous catecholamines —epinephrine (adrenaline) and norepinephrine (noradrenaline) in particular—on β-adrenergic receptors, part of the sympathetic nervous system that mediates the fight-or-flight response.
The basis of autonomic pharmacology reflects the physiology of the sympathetic nervous system (SNS) and the parasympathetic nervous system (PSNS) to regulate involuntary reactions to stresses on multiorgan systems within the body. When a pathologic process is present that affects the homeostasis achieved between the SNS and PSNS in this process, either of these branches can become overactive while the other is excessively inhibited.[rx] This break in homeostasis results in various clinical manifestations that can range in severity from simply presenting rhinorrhea symptomology to fatal presentations like cardiovascular collapse.[rx] For a wide range of presentations and severity of pathologies, the agents classified in autonomic pharmacology are indicated to re-establish the homeostasis that the human body attempts to produce via the autonomic nervous system (ANS).[rx]
Within autonomic pharmacology, there are four specific categories of drugs based on how they affect the ANS
-
Cholinomimetics/cholinesterase antagonists
-
Anticholinergics
-
Adrenoreceptor agonists/sympathomimetics
-
Adrenoreceptor antagonists
The clinical indications of medications from each of the four categories are listed below. Important to note is that this is not a complete list due to the vastness of this topic; the drugs included are representative of each category.
FDA-labeled indications
Cholinomimetics/Cholinesterase antagonists[rx][rx][rx][rx]
-
Bethanechol – postoperative and neurogenic ileus and urinary retention
-
Pilocarpine – glaucoma and alleviating the symptoms of Sjogren’s syndrome
-
Nicotine – found in smoking cessation regimens
-
Cholinesterase inhibitors (neostigmine, edrophonium, pyridostigmine, physostigmine) – the diagnosis and treatment of myasthenia gravis, maintenance treatment of Alzheimer disease, and specifically neostigmine used commonly with glycopyrrolate to reverse neuromuscular blockade in postoperative anesthesia practice
Anticholinergics[rx][rx][rx][rx][rx][rx][rx]:
-
Atropine – used in ACLS guidelines to correct bradyarrhythmias and in ophthalmic surgery as a retinal dilator
-
Ipratropium and tiotropium – correct acute exacerbations of bronchospasm (asthma, COPD), as well as exacerbation prophylaxis for those conditions
-
Scopolamine – prevents motion sickness and postoperative nausea/vomiting
-
Oxybutynin – urge incontinence and postoperative bladder spasm
-
Dicyclomine, glycopyrrolate – can be used for reducing diarrhea output in irritable bowel syndrome; glycopyrrolate can also be added to cholinesterase reversal of neuromuscular blockades in postoperative anesthesia care to prevent bronchospasm and is currently undergoing investigation as an adjunct treatment in COPD
Adrenoreceptor agonists/Sympathomimetics[rx][rx][rx][rx][rx][rx][rx]:
-
Clonidine – used as an antihypertensive
-
Dobutamine, phenylephrine, epinephrine – used to correct severe hypotension in cardiogenic shock and acute heart failure exacerbation; epinephrine specifically also used in ACLS guidelines for non-shockable heart rhythms in cardiac arrest and rapid reversal of fatal anaphylactic reactions
-
Albuterol – fast-acting bronchodilator used in acute asthma exacerbations
-
Fenoldopam – corrects hypertension
-
Bromocriptine – involved in the maintenance of Parkinson disease and conditions involving prolactinoma
Adrenoreceptor antagonists[rx][rx][rx]:
-
Phenoxybenzamine, phentolamine – used to correct high catecholamine states
-
Prazosin, doxazosin, terazosin, tamsulosin – indicated to correct urinary retention in benign prostatic hyperplasia
-
Beta-blockers (propranolol, metoprolol, labetalol, etc.) – indicated for many cardiovascular conditions since they are in the classification of class II antiarrhythmics; these agents are used to manage tachyarrhythmias, hypertension, angina, heart failure, and migraine prophylaxis
Mechanism of Action
As with the homeostasis established via processes performed by the SNS and PSNS, drugs from each of the four categories listed above also work inversely to each other. The primary mechanism of action for most of these agents are to serve as either agonists or antagonists of specific receptors within these systems.[rx] The receptors with their locations and physiologic actions are listed below.
For adrenoreceptors stimulated by norepinephrine (synapses) and epinephrine (endocrine), involved in SNS processes[rx][rx]:
-
Alpha-1 (A1) – located mostly in postsynaptic effector cells found in smooth muscle; effects mediated by IP3/DAG path, include mydriasis due to contraction of radial muscles, constriction of arteries and veins, urinary retention due to internal/external urethral sphincter contraction, and a decrease in renin release from renal juxtaglomerular cells
-
Alpha-2 (A2) – located in presynaptic adrenergic terminals found in lipocytes and smooth muscle; effects mediated by decreasing cAMP, including a decrease in norepinephrine release, stimulates platelet aggregation and decreases insulin secretion
-
Beta-1 (B1) – located in postsynaptic effector cells in the SA node of the heart, lipocytes, brain, juxtaglomerular apparatus of renal tubules, and the ciliary body epithelium; effects mediated by increasing cAMP, including increased heart rate and the conduction velocity through the cardiac nodes, also increases renin release from renal juxtaglomerular cells
-
Beta-2 (B2) – located in postsynaptic effector cells in smooth muscle and cardiac myocytes; effects mediated by increasing cAMP, include vasodilation, bronchiole dilation, increased insulin secretion, and uterine relaxation
-
Beta-3 (B3) – located in postsynaptic effector cells in lipocytes and myocardium; similar effects to beta-1 receptors mediated by increasing cAMP
For choline receptors stimulated by acetylcholine, most involved in PSNS processes[rx]
-
Muscarinic-1 (M1) – important to note is the only choline receptor involved in an SNS process, located in sweat glands of the skin; effects mediated by IP3/DAG path, include glandular contraction and increased secretion
-
Muscarinic-2 (M2) – located in SA and AV nodes and myocardium; effects mediated by decreasing cAMP, include decreasing heart rate and myocardial conduction velocity
-
Muscarinic-3 (M3) – located in the smooth muscle of various organ systems; effects mediated by IP3/DAG path, include contraction of the ciliary muscle causing miosis, contraction of bronchioles, increased bronchiole secretions, increased GI motility, detrusor muscle contraction, and internal/external urethral sphincter relaxation
-
Muscarinic-4 (M4) and Muscarinic (M5) – located primarily in the CNS, e.g., forebrain and substania nigra, respectively
-
Nicotinic-N (NN) – located in postsynaptic dendrites of both sympathetic and parasympathetic postganglionic neurons; effects mediated by Na+/K+ depolarization, include increased neurotransmission
-
Nicotinic-M (NM) – located in neuromuscular endplates of skeletal muscle; effects mediated by Na+/K+ depolarization, include skeletal muscle contraction
For dopamine receptors, most involved in both SNS and PSNS processes[rx]:
-
Dopamine 1-5 (D1-5) – located in the CNS, except for Dopamine-1 receptors, which also appear in renal vasculature; effects mediated by cAMP path, include renal artery vasodilation, increased renal blood flow, and modulation of neuroendocrine signaling
In terms of the four categories mentioned, each is an agonist and/or antagonist of the receptors listed. Cholinomimetics have agonist activity at muscarinic receptors augmenting PSNS activity to achieve the desired effects of increasing GI motility and decreasing intraocular pressure.[rx][rx] Whereas the other agents mentioned work directly on receptors as agonists/antagonists, the subcategory of drugs that also achieve similar effects to cholinomimetics is the cholinesterase antagonists. These agents inhibit acetylcholinesterase enzymes within the synaptic cleft to increase the concentration of acetylcholine, resulting in increased PSNS neurotransmission and facilitating skeletal muscle contraction.[rx] Inversely, the anticholinergic agents work to inhibit PSNS activity, the main mechanism of action involving antagonism of muscarinic receptors resulting in increased heart rate and conduction velocity and stimulate bronchodilation.[rx][rx]
Within the SNS system, adrenoreceptor agonists/sympathomimetics work at alpha and beta receptors to potentiate SNS activity to achieve higher cardiac output and fast bronchodilation.[rx][rx] Inversely, adrenoreceptor antagonists are also active at alpha and beta receptors in decreasing SNS neurotransmission to reduce heart rate, dampen high catecholamine states, and increase urinary smooth muscle relaxation.[rx][rx][23]
Administration
Most agents are available as IV, IM, SC, PO formulations.[rx][rx][rx] Some agents can also be given topically as eye drops, specific to ophthalmologic surgery requiring extended pupillary dilation and the medical treatment of open-angle and closed-angle glaucoma.[rx][rx]
Adverse Effects
Due to the various effects of the ANS on cardiovascular, pulmonary, gastrointestinal, and genitourinary systems, the general theme of reactions to these medications involves effects on these organ systems. The various reactions to each of the categories of agents include[rx][rx][rx][rx][rx]:
-
Cholinomimetics/cholinesterase inhibitors – nausea, vomiting, diarrhea, urinary urgency, excessive salivation, sweating, cutaneous vasodilation, bronchial constriction
-
Anticholinergics – tachycardia, urinary retention, xerostomia (dry mouth), constipation, increased intraocular pressure
-
Adrenoreceptor agonists/sympathomimetics – tremor, tachycardia, hypertension, urinary retention, piloerection
-
Adrenoreceptor antagonists – bradycardia, bronchospasm, hypotension
Contraindications
Based on the adverse reaction profiles of each category, several significant contraindications can be elucidated[rx][rx][rx]:
-
Cholinomimetics/Cholinesterase inhibitors – relative contraindications in asthma/COPD, bradycardia, volume-depleted/hypotension, cardiogenic shock, sepsis, reduced ejection fraction heart failure
-
Anticholinergics – relative contraindications in glaucoma especially angle-closure, older men with benign prostatic hyperplasia, and peptic ulcer disease; atropine specifically not recommended for children, especially infants who are sensitive to its hyperthermic effects
-
Adrenoreceptor agonists/Sympathomimetics – relative contraindications in patients with a previous/current history of tachycardia or hypokalemia, hypertension, urinary retention, gastroparesis; for clonidine specifically in elderly who are more prone to fall from orthostatic hypotension, and epinephrine in those with angle-closure glaucoma
-
Adrenoreceptor antagonists – relative contraindications for alpha-blockers in orthostatic hypotension, tachycardia, myocardial ischemia; for beta-blockers asthma/COPD for the nonselective agents, bradycardia, hypotension
Toxicity
Toxic profiles of the four categories described are mostly involved in overdose, exhibiting the same effects that are augmented so that the benefits no longer outweigh the risks. The primary reversal strategy for these situations typically is to discontinue the offending agent and treat the resultant symptoms.[rx] Several agents of each category have toxic effects which require more specific reversal methods as listed[rx][rx][rx][rx]:
-
Cholinesterase inhibitors (neostigmine, pyridostigmine, physostigmine) – formerly, high doses of these agents were used in chemical warfare would present as miosis, bronchial constriction, vomiting and diarrhea, and progress to convulsions, coma, and finally death; this toxicity profile remains the same and can be reversed with pralidoxime with adjunctive parenteral atropine and benzodiazepines for possible seizure activity
-
Atropine – can cause vision disturbances when in excess resulting in prolonged mydriasis and cycloplegia, can also exacerbate closed-angle glaucoma by increasing intraocular pressure; reversal generally is to discontinue; however, physostigmine has utility in extreme cases such as severe elevation of body temperature and rapid supraventricular tachycardia
-
Clonidine – can cause xerostomia and sedation; though currently there is no approved reversal, studies are currently investigating the use of naloxone as a reversal agent
-
Beta-blockers – besides severe hypotension and bradycardia, tremors and bronchospasm are worrisome in the event of overdose; glucagon serves as the reversal agent
Enhancing Healthcare Team Outcomes
Healthcare professionals who prescribe medications that work on the autonomic system must be fully aware of the side effects of these agents. Requisite close monitoring of vital signs, including blood pressure, heart rate, respiratory rate, oxygen saturation, and temperature is strongly recommended when attempting to reestablish autonomic homeostasis with ANS agents.[rx] Several common conditions which require autonomic pharmacological correction need specific monitoring[rx][rx][rx][rx]:
-
Glaucoma – ocular telemetry sensors can help to continuously monitor intraocular pressure.
-
Shock – requires several monitoring functions as listed:
-
Maintaining a MAP of 65 and above
-
MAP measurements via an arterial line
-
Pulse pressure variation to guide fluid therapy
-
Bedside echocardiography to assess chambers of the heart and looking for cardiogenic shock vs. obstructive shock (massive PE) and calculate cardiac output/ejection fraction
-
Pulse index continuous cardiac output (PiCCO) device which can serve to continuously monitor continuous cardiac output and assess fluid response
-
-
Asthma/COPD – pulmonary function testing is the standard to diagnose and monitor the severity of pulmonary obstruction; can also evaluate the effectiveness of inhaled autonomic agents in reversing obstructive processes
-
Arrhythmias – for acute monitoring 4-lead ECG and 12-lead EKG are standard for monitoring tachycardias or bradycardias; if extended monitoring is required extended continuous ambulatory rhythm monitors (ECAM) is the monitoring modality of choice
Physicians, nurses, and pharmacists need to work collaboratively when using medications that interact with the autonomic nervous system to make sure that the pharmacotherapy is safe and effective for each patient.
References

