Esophageal Thrush/Esophageal candidiasis (EC) is one of the most common opportunistic infections in patients with impaired cellular immunity, and it is and the most common gastrointestinal (GI) opportunistic disorder among individuals infected with human immunodeficiency virus (HIV) [rx], [rx] However, we have sometimes come across EC in healthy individuals without HIV infection.[rx–rx] When EC has been found in healthy individuals, predisposing medical conditions have often been identified.[rx–rx] Broad-spectrum antibiotics may eliminate certain bacteria that inhibit fungal growth, thereby enhancing Candida overgrowth.[rx] Soon after the introduction of H2-receptor antagonists, some isolated cases of digestive Candida were reported.[rx]
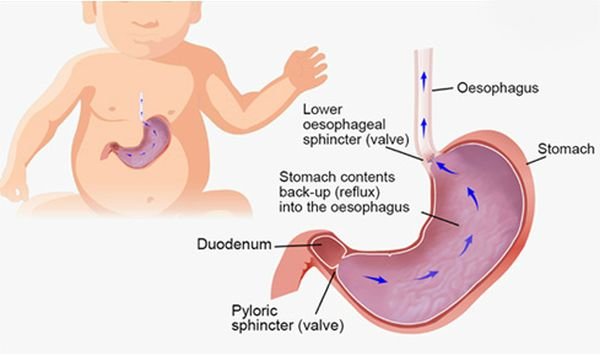
Causes of Esophageal Thrush
- Invasive candidiasis – is caused by 15 of the more than 150 known species of Candida. These species, all confirmed by isolation from patients, are: C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, C. krusei, C. guilliermondii, C. lusitaniae, C. dubliniensis, C. pelliculosa, C. kefyr, C. lipolytica, C. famata, C. inconspicua, C. rugosa, and C. norvegensis. Over the last 20 – 30 years, C. albicans has been responsible for 95% of infections, with, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei causing the majority of the remaining cases.[rx]
- Recently,– C. auris, a species first reported in 2009, has been found to cause invasive candidiasis. C. auris has attracted attention because it can be resistant to the antifungal medications used to treat candidiasis.[rx]
- Weakened immunity. Oral thrush is more likely to occur in infants and older adults due to reduced immunity. Some medical conditions and treatments can suppress your immune system, such as cancer and its treatments, organ transplantation and required drugs that suppress the immune system, and HIV/AIDS.
- Diabetes. If you have untreated diabetes or the disease isn’t well-controlled, your saliva may contain large amounts of sugar, which encourages the growth of candida.
- Vaginal yeast infections. Vaginal yeast infections are caused by the same fungus that causes oral thrush. You can pass the infection to your baby.
- Medications. Drugs such as prednisone, inhaled corticosteroids, or antibiotics that disturb the natural balance of microorganisms in your body can increase your risk of oral thrush.
- Other oral conditions. Wearing dentures, especially upper dentures, or having conditions that cause dry mouth can increase the risk of oral thrush.
Symptoms of Esophageal Thrush
The symptoms of esophageal thrush include
- Some patients present with esophageal candidiasis as a first presentation of systemic candidiasis.
- white lesions on the lining of your esophagus that may look like cottage cheese and may bleed if they’re scraped
- People with esophageal candidiasis typically present with difficult or painful swallowing. Longstanding esophageal candidiasis can result in weight loss. There is often concomitant thrush in the mouth.
- Pain or discomfort when swallowing
- Dry mouth
- Difficulty swallowing
- Nausea
- Vomiting
- Weight loss
- Chest pain
- Creamy white patches on the inside of the cheeks and on surface of the tongue
- White lesions on the roof of your mouth, tonsils, and gums
- Cracking in the corner of your mouth
- Especially red, sensitive, cracking, or itchy nipples
- Stabbing pains felt deep within the breast
- Significant pain when nursing or pain between nursing sessions
Diagnosis of Esophageal Thrush
These include
- Endoscopic exam – In this procedure, your doctor examines your esophagus, stomach and upper part of your small intestine (duodenum) using a lighted, flexible tube with a camera on the tip (endoscope).
- Physical exam – If needed, a physical exam and certain blood tests may be done to try to identify any possible underlying medical condition that could cause thrush in the esophagus.
- Upper endoscopy – A test in which a long, flexible lighted tube, called an endoscope, is used to view the esophagus.
- Biopsy – During this test, a small sample of the esophageal tissue is removed and then sent to a laboratory to be examined under a microscope.
- Upper GI series (or barium swallow) – During this procedure, X-rays are taken of the esophagus after drinking a barium solution. Barium coats the lining of the esophagus and shows up white on an X-ray. This characteristic enables doctors to view certain abnormalities of the esophagus.
Treatment of Esophageal Candidiasis
The current first-line treatment is fluconazole, 200 mg. on the first day, followed by daily dosing of 100 mg. for at least 21 days total. Treatment should continue for 14 days after relief of symptoms. Other therapy options include:
- Treatment of esophageal candidiasis involves the use of antifungal therapy. Unlike oropharyngeal candidiasis, esophageal candidiasis should always have therapy with systemic agents and not topical agents.
- The most commonly used medication to treat esophageal candidiasis is oral fluconazole 200 to 400 mg per day for 14 to 21 days.
- If patients cannot tolerate oral intake, then intravenous Fluconazole 400 mg daily can be used and then deescalated to oral Fluconazole when the patient can tolerate oral medications. Fluconazole 100 to 200 mg three times per week can be used to suppress recurrent esophageal candidiasis. Micafungin 150 mg IV daily has been shown to be non-inferior to fluconazole at 200 mg daily.
- Itraconazole 200 mg per day orally or Voriconazole 200 mg twice daily for 14 to 21 days are other treatment options.
- Amphotericin B deoxycholate 0.3 to 0.7 mg/kg daily can be used in patients with refractory candida esophagitis, but it has serious medication side effects and should be avoided if possible. Posaconazole 400 mg twice daily has been effective in refractory esophageal candidiasis as well. [rx][rx][rx]
- Since esophageal candidiasis is an opportunistic infection and most often seen in immunocompromised persons, the cause of the immunosuppression should be diagnosed and treated as well.[rx][rx][rx]
- nystatin is not an effective treatment for esophageal candidiasis. It can be used as (swish, do not swallow) treatment for oral candidiasis that occurs with the use of asthma pumps.
- other oral triazoles, such as itraconazole
- caspofungin, used in refractory or systemic cases
- amphotericin, used in refractory or systemic cases
[dropshadowbox align=”none” effect=”lifted-both” width=”auto” height=”” background_color=”#ffffff” border_width=”1″ border_color=”#dddddd” ]
Antifungals for oropharyngeal candidiasis
| Antifungal agent | Form | Strength | Usage | Cost |
|---|---|---|---|---|
| Topical | ||||
| Nystatin | Pastille | 200 000 units | 1–2 pastilles, 4 times daily | + |
| Suspension | 5 mL swish-and-Swallow, 4 times daily | + | ||
| + | ||||
| Clotrimazole | Oral troche | 10 mg troche | Dissolve 1 troche, 5 times daily | + |
| Amphotericin B | Suspension | 1 mg/mL | 1 mL swish-and-swallow, 4 times daily | + |
| Lozenge | 100 mg | Four times daily | + | |
| Tablet | 10 mg | Four times daily | + | |
| Miconazole (Lauriad®) | Lauriad | 10 mg | Apply to gum once daily | TBD |
| Systemic | ||||
| Ketoconazole | Tablet | 200 mg | 1–2 tablets, once to twice daily | + |
| Fluconazole | Tablet | 100 mg | 1 tablet daily | ++ |
| Solution | 10 mg/mL | 10 mL, once daily | ++ | |
| Itraconazole | Capsule | 100 mg | 2 capsules, once daily | ++ |
| Solution | 10 mg/mL | 10–20 mL, once daily | ++ | |
| Posaconazole | Suspension | 100 mg/2.5 mL | 2 tsp daily | +++ |
Antifungals for esophageal candidiasis
| Antifungal agent | Form | Strength | Usage |
|---|---|---|---|
| Azoles | |||
| Ketoconazole | Tablets | 200 mg | 1–2 tablets, once to twice daily |
| Fluconazole | Tablet | 100 mg | 1 tablet daily |
| SolutionIV piggyback | 10 mg/mL | 10 mL, once daily 100 mg, once daily |
|
| Itraconazole | Capsule | 100 mg | 2 capsules, once daily |
| Solution | 10 mg/mL | 20 mL, once daily | |
| Posaconazole | Suspension | 100 mg/2.5 mL | 4 tsp, twice daily |
| Voriconazole | Tablet/IV piggyback | 200 mg | Once daily |
| Caspofungin | Intravenous | 50 mg | Once daily |
| Micafungin | Intravenous | 150 mg | Once daily |
| Anidulafungin | Intravenous | 50 mg | Once daily |
| Amphotericin B | Intravenous | 0.3–0.7 mg/kg | Once daily |
[/dropshadowbox]
Treatment for Recommendations
- An echinocandin (caspofungin: loading dose 70 mg, then 50 mg daily; micafungin: 100 mg daily; anidulafungin: loading dose 200 mg, then 100 mg daily) is recommended as initial therapy (strong recommendation; high-quality evidence).
- Fluconazole, intravenous or oral, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily is an acceptable alternative to an echinocandin as initial therapy in selected patients, including those who are not critically ill and who are considered unlikely to have a fluconazole-resistant Candida species (strong recommendation; high-quality evidence).
- Testing for azole susceptibility is recommended for all bloodstream and other clinically relevant Candida isolates. Testing for echinocandin susceptibility should be considered in patients who have had prior treatment with an echinocandin and among those who have infection with C. glabrata or C. parapsilosis (strong recommendation; low-quality evidence).
- Transition from an echinocandin to fluconazole (usually within 5–7 days) is recommended for patients who are clinically stable, have isolates that are susceptible to fluconazole (eg, C. albicans), and have negative repeat blood cultures following initiation of antifungal therapy (strong recommendation; moderate-quality evidence).
- For infection due to C. glabrata, transition to higher-dose fluconazole 800 mg (12 mg/kg) daily or voriconazole 200–300 (3–4 mg/kg) twice daily should only be considered among patients with fluconazole-susceptible or voriconazole-susceptible isolates (strong recommendation; low-quality evidence).
- Lipid formulation amphotericin B (AmB) (3–5 mg/kg daily) is a reasonable alternative if there is intolerance, limited availability, or resistance to other antifungal agents (strong recommendation; high-quality evidence).
- Transition from AmB to fluconazole is recommended after 5–7 days among patients who have isolates that are susceptible to fluconazole, who are clinically stable, and in whom repeat cultures on antifungal therapy are negative (strong recommendation; high-quality evidence).
- Among patients with suspected azole- and echinocandin-resistant Candidainfections, lipid formulation AmB (3–5 mg/kg daily) is recommended (strong recommendation; low-quality evidence).
- Voriconazole 400 mg (6 mg/kg) twice daily for 2 doses, then 200 mg (3 mg/kg) twice daily is effective for candidemia, but offers little advantage over fluconazole as initial therapy (strong recommendation; moderate-quality evidence).Voriconazole is recommended as step-down oral therapy for selected cases of candidemia due to C. krusei (strong recommendation; low-quality evidence).
- All nonneutropenic patients with candidemia should have a dilated ophthalmological examination, preferably performed by an ophthalmologist, within the first week after diagnosis (strong recommendation; low-quality evidence).
- Follow-up blood cultures should be performed every day or every other day to establish the time point at which candidemia has been cleared (strong recommendation; low-quality evidence).
- Recommended duration of therapy for candidemia without obvious metastatic complications is for 2 weeks after documented clearance of Candida species from the bloodstream and resolution of symptoms attributable to candidemia (strong recommendation; moderate-quality evidence).
II. Should Central Venous Catheters Be Removed in Nonneutropenic Patients With Candidemia?
Recommendation
- Central venous catheters (CVCs) should be removed as early as possible in the course of candidemia when the source is presumed to be the CVC and the catheter can be removed safely; this decision should be individualized for each patient (strong recommendation; moderate-quality evidence).
III. What Is the Treatment for Candidemia in Neutropenic Patients?
Recommendations
- An echinocandin (caspofungin: loading dose 70 mg, then 50 mg daily; micafungin: 100 mg daily; anidulafungin: loading dose 200 mg, then 100 mg daily) is recommended as initial therapy (strong recommendation; moderate-quality evidence).
- Lipid formulation AmB, 3–5 mg/kg daily, is an effective but less attractive alternative because of the potential for toxicity (strong recommendation; moderate-quality evidence).
- Fluconazole, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily, is an alternative for patients who are not critically ill and have had no prior azole exposure (weak recommendation; low-quality evidence).
- Fluconazole, 400 mg (6 mg/kg) daily, can be used for step-down therapy during persistent neutropenia in clinically stable patients who have susceptible isolates and documented bloodstream clearance (weak recommendation; low-quality evidence).
- Voriconazole, 400 mg (6 mg/kg) twice daily for 2 doses, then 200–300 mg (3–4 mg/kg) twice daily, can be used in situations in which additional mold coverage is desired (weak recommendation; low-quality evidence). Voriconazole can also be used as step-down therapy during neutropenia in clinically stable patients who have had documented bloodstream clearance and isolates that are susceptible to voriconazole (weak recommendation; low-quality evidence).
- For infections due to C. krusei, an echinocandin, lipid formulation AmB, or voriconazole is recommended (strong recommendation; low-quality evidence).
- Recommended minimum duration of therapy for candidemia without metastatic complications is 2 weeks after documented clearance of Candida from the bloodstream, provided neutropenia and symptoms attributable to candidemia have resolved (strong recommendation; low-quality evidence).
- Ophthalmological findings of choroidal and vitreal infection are minimal until recovery from neutropenia; therefore, dilated funduscopic examinations should be performed within the first week after recovery from neutropenia (strong recommendation; low-quality evidence).
- In the neutropenic patient, sources of candidiasis other than a CVC (eg, gastrointestinal tract) predominate. Catheter removal should be considered on an individual basis (strong recommendation; low-quality evidence).
- Granulocyte colony – stimulating factor (G-CSF)–mobilized granulocyte transfusions can be considered in cases of persistent candidemia with anticipated protracted neutropenia (weak recommendation; low-quality evidence).
IV. What Is the Treatment for Chronic Disseminated (Hepatosplenic) Candidiasis?
Recommendations
- Initial therapy with lipid formulation AmB, 3–5 mg/kg daily OR an echinocandin (micafungin: 100 mg daily; caspofungin: 70-mg loading dose, then 50 mg daily; or anidulafungin: 200-mg loading dose, then 100 mg daily), for several weeks is recommended, followed by oral fluconazole, 400 mg (6 mg/kg) daily, for patients who are unlikely to have a fluconazole-resistant isolate (strong recommendation; low-quality evidence).
- Therapy should continue until lesions resolve on repeat imaging, which is usually several months. Premature discontinuation of antifungal therapy can lead to relapse (strong recommendation; low-quality evidence).
- If chemotherapy or hematopoietic cell transplantation is required, it should not be delayed because of the presence of chronic disseminated candidiasis, and antifungal therapy should be continued throughout the period of high risk to prevent relapse (strong recommendation; low-quality evidence).
- For patients who have debilitating persistent fevers, short-term (1–2 weeks) treatment with nonsteroidal anti-inflammatory drugs or corticosteroids can be considered (weak recommendation; low-quality evidence).
V. What Is the Role of Empiric Treatment for Suspected Invasive Candidiasis in Nonneutropenic Patients in the Intensive Care Unit?
Recommendations
- Empiric antifungal therapy should be considered in critically ill patients with risk factors for invasive candidiasis and no other known cause of fever and should be based on clinical assessment of risk factors, surrogate markers for invasive candidiasis, and/or culture data from nonsterile sites (strong recommendation; moderate-quality evidence). Empiric antifungal therapy should be started as soon as possible in patients who have the above risk factors and who have clinical signs of septic shock (strong recommendation; moderate-quality evidence).
- Preferred empiric therapy for suspected candidiasis in nonneutropenic patients in the intensive care unit (ICU) is an echinocandin (caspofungin: loading dose of 70 mg, then 50 mg daily; micafungin: 100 mg daily; anidulafungin: loading dose of 200 mg, then 100 mg daily) (strong recommendation; moderate-quality evidence).
- Fluconazole, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily, is an acceptable alternative for patients who have had no recent azole exposure and are not colonized with azole-resistant Candida species (strong recommendation; moderate-quality evidence).
- Lipid formulation AmB, 3–5 mg/kg daily, is an alternative if there is intolerance to other antifungal agents (strong recommendation; low-quality evidence).
- Recommended duration of empiric therapy for suspected invasive candidiasis in those patients who improve is 2 weeks, the same as for treatment of documented candidemia (weak recommendation; low-quality evidence).
- For patients who have no clinical response to empiric antifungal therapy at 4–5 days and who do not have subsequent evidence of invasive candidiasis after the start of empiric therapy or have a negative non-culture-based diagnostic assay with a high negative predictive value, consideration should be given to stopping antifungal therapy (strong recommendation; low-quality evidence).
VI. Should Prophylaxis Be Used to Prevent Invasive Candidiasis in the Intensive Care Unit Setting?
Recommendations
- Fluconazole, 800-mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily, could be used in high-risk patients in adult ICUs with a high rate (>5%) of invasive candidiasis (weak recommendation; moderate-quality evidence).
- An alternative is to give an echinocandin (caspofungin: 70-mg loading dose, then 50 mg daily; anidulafungin: 200-mg loading dose and then 100 mg daily; or micafungin: 100 mg daily) (weak recommendation; low-quality evidence).
- Daily bathing of ICU patients with chlorhexidine, which has been shown to decrease the incidence of bloodstream infections including candidemia, could be considered (weak recommendation; moderate-quality evidence).
VII. What Is the Treatment for Neonatal Candidiasis, Including Central Nervous System Infection?
What Is the Treatment for Invasive Candidiasis and Candidemia?
Recommendations
- AmB deoxycholate, 1 mg/kg daily, is recommended for neonates with disseminated candidiasis (strong recommendation; moderate-quality evidence).
- Fluconazole, 12 mg/kg intravenous or oral daily, is a reasonable alternative in patients who have not been on fluconazole prophylaxis (strong recommendation; moderate-quality evidence).
- Lipid formulation AmB, 3–5 mg/kg daily, is an alternative, but should be used with caution, particularly in the presence of urinary tract involvement (weak recommendation; low-quality evidence).
- Echinocandins should be used with caution and generally limited to salvage therapy or to situations in which resistance or toxicity preclude the use of AmB deoxycholate or fluconazole (weak recommendation; low-quality evidence).
- A lumbar puncture and a dilated retinal examination are recommended in neonates with cultures positive for Candida species from blood and/or urine (strong recommendation; low-quality evidence).
- Computed tomographic or ultrasound imaging of the genitourinary tract, liver, and spleen should be performed if blood cultures are persistently positive for Candida species (strong recommendation; low-quality evidence).
- CVC removal is strongly recommended (strong recommendation; moderate-quality evidence).
- The recommended duration of therapy for candidemia without obvious metastatic complications is for 2 weeks after documented clearance of Candidaspecies from the bloodstream and resolution of signs attributable to candidemia (strong recommendation; low-quality evidence).
What Is the Treatment for Central Nervous System Infections in Neonates?
Recommendations
- For initial treatment, AmB deoxycholate, 1 mg/kg intravenous daily, is recommended (strong recommendation; low-quality evidence).
- An alternative regimen is liposomal AmB, 5 mg/kg daily (strong recommendation; low-quality evidence).
- The addition of flucytosine, 25 mg/kg 4 times daily, may be considered as salvage therapy in patients who have not had a clinical response to initial AmB therapy, but adverse effects are frequent (weak recommendation; low-quality evidence).
- For step-down treatment after the patient has responded to initial treatment, fluconazole, 12 mg/kg daily, is recommended for isolates that are susceptible to fluconazole (strong recommendation; low-quality evidence).
- Therapy should continue until all signs, symptoms, and cerebrospinal fluid (CSF) and radiological abnormalities, if present, have resolved (strong recommendation; low-quality evidence).
- Infected central nervous system (CNS) devices, including ventriculostomy drains and shunts, should be removed if at all possible (strong recommendation; low-quality evidence).
What Are the Recommendations for Prophylaxis in the Neonatal Intensive Care Unit Setting?
Recommendations
- In nurseries with high rates (>10%) of invasive candidiasis, intravenous or oral fluconazole prophylaxis, 3–6 mg/kg twice weekly for 6 weeks, in neonates with birth weights <1000 g is recommended (strong recommendation; high-quality evidence)
- Oral nystatin, 100 000 units 3 times daily for 6 weeks, is an alternative to fluconazole in neonates with birth weights <1500 g in situations in which availability or resistance preclude the use of fluconazole (weak recommendation; moderate-quality evidence).
- Oral bovine lactoferrin (100 mg/day) may be effective in neonates <1500 g but is not currently available in US hospitals (weak recommendation; moderate-quality evidence).
VIII. What Is the Treatment for Intra-abdominal Candidiasis?
Recommendations
- Empiric antifungal therapy should be considered for patients with clinical evidence of intra-abdominal infection and significant risk factors for candidiasis, including recent abdominal surgery, anastomotic leaks, or necrotizing pancreatitis (strong recommendation; moderate-quality evidence).
- Treatment of intra-abdominal candidiasis should include source control, with appropriate drainage and/or debridement (strong recommendation; moderate-quality evidence).
- The choice of antifungal therapy is the same as for the treatment of candidemia or empiric therapy for nonneutropenic patients in the ICU (See sections I and V) (strong recommendation; moderate-quality evidence).
- The duration of therapy should be determined by adequacy of source control and clinical response (strong recommendation; low-quality evidence).
IX. Does the Isolation of Candida Species From the Respiratory Tract Require Antifungal Therapy?
Recommendation
- Growth of Candida from respiratory secretions usually indicates colonization and rarely requires treatment with antifungal therapy (strong recommendation; moderate-quality evidence).
X. What Is the Treatment for Candida Intravascular Infections, Including Endocarditis and Infections of Implantable Cardiac Devices?
What Is the Treatment for Candida Endocarditis?
Recommendations
- For native valve endocarditis, lipid formulation AmB, 3–5 mg/kg daily, with or without flucytosine, 25 mg/kg 4 times daily, OR high-dose echinocandin (caspofungin 150 mg daily, micafungin 150 mg daily, or anidulafungin 200 mg daily) is recommended for initial therapy (strong recommendation; low-quality evidence).
- Step-down therapy to fluconazole, 400–800 mg (6–12 mg/kg) daily, is recommended for patients who have susceptible Candida isolates, have demonstrated clinical stability, and have cleared Candida from the bloodstream (strong recommendation; low-quality evidence).
- Oral voriconazole, 200–300 mg (3–4 mg/kg) twice daily, or posaconazole tablets, 300 mg daily, can be used as step-down therapy for isolates that are susceptible to those agents but not susceptible to fluconazole (weak recommendation; very low-quality evidence).
- Valve replacement is recommended; treatment should continue for at least 6 weeks after surgery and for a longer duration in patients with perivalvular abscesses and other complications (strong recommendation; low-quality evidence).
- For patients who cannot undergo valve replacement, long-term suppression with fluconazole, 400–800 mg (6–12 mg/kg) daily, if the isolate is susceptible, is recommended (strong recommendation; low-quality evidence).
- For prosthetic valve endocarditis, the same antifungal regimens suggested for native valve endocarditis are recommended (strong recommendation; low-quality evidence). Chronic suppressive antifungal therapy with fluconazole, 400–800 mg (6–12 mg/kg) daily, is recommended to prevent recurrence (strong recommendation; low-quality evidence).
What Is the Treatment for Candida Infection of Implantable Cardiac Devices?
Recommendations
- For pacemaker and implantable cardiac defibrillator infections, the entire device should be removed (strong recommendation; moderate-quality evidence).
- Antifungal therapy is the same as that recommended for native valve endocarditis (strong recommendation; low-quality evidence).
- For infections limited to generator pockets, 4 weeks of antifungal therapy after removal of the device is recommended (strong recommendation; low-quality evidence).
- For infections involving the wires, at least 6 weeks of antifungal therapy after wire removal is recommended (strong recommendation; low-quality evidence).
- For ventricular assist devices that cannot be removed, the antifungal regimen is the same as that recommended for native valve endocarditis (strong recommendation; low-quality evidence). Chronic suppressive therapy with fluconazole if the isolate is susceptible, for as long as the device remains in place is recommended (strong recommendation; low-quality evidence).
What Is the Treatment for Candida Suppurative Thrombophlebitis?
Recommendations
- Catheter removal and incision and drainage or resection of the vein, if feasible, is recommended (strong recommendation; low-quality evidence).
- Lipid formulation AmB, 3–5 mg/kg daily, OR fluconazole, 400–800 mg (6–12 mg/kg) daily, OR an echinocandin (caspofungin 150 mg daily, micafungin 150 mg daily, or anidulafungin 200 mg daily) for at least 2 weeks after candidemia (if present) has cleared is recommended (strong recommendation; low-quality evidence).
- Step-down therapy to fluconazole, 400–800 mg (6–12 mg/kg) daily, should be considered for patients who have initially responded to AmB or an echinocandin, are clinically stable, and have a fluconazole-susceptible isolate (strong recommendation; low-quality evidence).
- Resolution of the thrombus can be used as evidence to discontinue antifungal therapy if clinical and culture data are supportive (strong recommendation; low-quality evidence).
XI. What Is the Treatment for Candida Osteoarticular Infections?
What Is the Treatment for Candida Osteomyelitis?
Recommendations
- Fluconazole, 400 mg (6 mg/kg) daily, for 6–12 months OR an echinocandin (caspofungin 50–70 mg daily, micafungin 100 mg daily, or anidulafungin 100 mg daily) for at least 2 weeks followed by fluconazole, 400 mg (6 mg/kg) daily, for 6–12 months is recommended (strong recommendation; low-quality evidence).
- Lipid formulation AmB, 3–5 mg/kg daily, for at least 2 weeks followed by fluconazole, 400 mg (6 mg/kg) daily, for 6–12 months is a less attractive alternative (weak recommendation; low-quality evidence).
- Surgical debridement is recommended in selected cases (strong recommendation; low-quality evidence).
What Is the Treatment for Candida Septic Arthritis?
- Fluconazole, 400 mg (6 mg/kg) daily, for 6 weeks OR an echinocandin (caspofungin 50–70 mg daily, micafungin 100 mg daily, or anidulafungin 100 mg daily) for 2 weeks followed by fluconazole, 400 mg (6 mg/kg) daily, for at least 4 weeks is recommended (strong recommendation; low-quality evidence)
- Lipid formulation AmB, 3–5 mg/kg daily, for 2 weeks, followed by fluconazole, 400 mg (6 mg/kg) daily, for at least 4 weeks is a less attractive alternative (weak recommendation; low-quality evidence).
- Surgical drainage is indicated in all cases of septic arthritis (strong recommendation; moderate-quality evidence).
- For septic arthritis involving a prosthetic device, device removal is recommended (strong recommendation; moderate-quality evidence).
- If the prosthetic device cannot be removed, chronic suppression with fluconazole, 400 mg (6 mg/kg) daily, if the isolate is susceptible, is recommended (strong recommendation; low-quality evidence).
XII. What Is the Treatment for Candida Endophthalmitis?
What Is the General Approach to Candida Endophthalmitis?
Recommendations
- All patients with candidemia should have a dilated retinal examination, preferably performed by an ophthalmologist, within the first week of therapy in nonneutropenic patients to establish if endophthalmitis is present (strong recommendation; low-quality evidence). For neutropenic patients, it is recommended to delay the examination until neutrophil recovery (strong recommendation; low-quality evidence).
- The extent of ocular infection (chorioretinitis with or without macular involvement and with or without vitritis) should be determined by an ophthalmologist (strong recommendation; low-quality evidence).
- Decisions regarding antifungal treatment and surgical intervention should be made jointly by an ophthalmologist and an infectious diseases physician (strong recommendation; low-quality evidence).
What Is the Treatment for Candida Chorioretinitis Without Vitritis?
Recommendations
- For fluconazole-/voriconazole-susceptible isolates, fluconazole, loading dose, 800 mg (12 mg/kg), then 400–800 mg (6–12 mg/kg) daily OR voriconazole, loading dose 400 mg (6 mg/kg) intravenous twice daily for 2 doses, then 300 mg (4 mg/kg) intravenous or oral twice daily is recommended (strong recommendation; low-quality evidence).
- For fluconazole-/voriconazole-resistant isolates, liposomal AmB, 3–5 mg/kg intravenous daily, with or without oral flucytosine, 25 mg/kg 4 times daily is recommended (strong recommendation; low-quality evidence).
- With macular involvement, antifungal agents as noted above PLUS intravitreal injection of either AmB deoxycholate, 5–10 µg/0.1 mL sterile water, or voriconazole, 100 µg/0.1 mL sterile water or normal saline, to ensure a prompt high level of antifungal activity is recommended (strong recommendation; low-quality evidence).
- The duration of treatment should be at least 4–6 weeks, with the final duration depending on resolution of the lesions as determined by repeated ophthalmological examinations (strong recommendation; low-quality evidence).
What Is the Treatment for Candida Chorioretinitis With Vitritis?
Recommendations
- Antifungal therapy as detailed above for chorioretinitis without vitritis, PLUS intravitreal injection of either amphotericin B deoxycholate, 5–10 µg/0.1 mL sterile water, or voriconazole, 100 µg/0.1 mL sterile water or normal saline is recommended (strong recommendation; low-quality evidence).
- Vitrectomy should be considered to decrease the burden of organisms and to allow the removal of fungal abscesses that are inaccessible to systemic antifungal agents (strong recommendation; low-quality evidence).
- The duration of treatment should be at least 4–6 weeks, with the final duration dependent on resolution of the lesions as determined by repeated ophthalmological examinations (strong recommendation; low-quality evidence).
XIII. What Is the Treatment for Central Nervous System Candidiasis?
Recommendations
- For initial treatment, liposomal AmB, 5 mg/kg daily, with or without oral flucytosine, 25 mg/kg 4 times daily is recommended (strong recommendation; low-quality evidence).
- For step-down therapy after the patient has responded to initial treatment, fluconazole, 400–800 mg (6–12 mg/kg) daily, is recommended (strong recommendation; low-quality evidence).
- Therapy should continue until all signs and symptoms and CSF and radiological abnormalities have resolved (strong recommendation; low-quality evidence).
- Infected CNS devices, including ventriculostomy drains, shunts, stimulators, prosthetic reconstructive devices, and biopolymer wafers that deliver chemotherapy should be removed if possible (strong recommendation; low-quality evidence).
- For patients in whom a ventricular device cannot be removed, AmB deoxycholate could be administered through the device into the ventricle at a dosage ranging from 0.01 mg to 0.5 mg in 2 mL 5% dextrose in water (weak recommendation; low-quality evidence).
XIV. What Is the Treatment for Urinary Tract Infections Due to Candida Species?
What Is the Treatment for Asymptomatic Candiduria?
Recommendations
- Elimination of predisposing factors, such as indwelling bladder catheters, is recommended whenever feasible (strong recommendation; low-quality evidence).
- Treatment with antifungal agents is NOT recommended unless the patient belongs to a group at high risk for dissemination; high-risk patients include neutropenic patients, very low-birth-weight infants (<1500 g), and patients who will undergo urologic manipulation (strong recommendation; low-quality evidence).
- Neutropenic patients and very low–birth-weight infants should be treated as recommended for candidemia (see sections III and VII) (strong recommendation; low-quality evidence).
- Patients undergoing urologic procedures should be treated with oral fluconazole, 400 mg (6 mg/kg) daily, OR AmB deoxycholate, 0.3–0.6 mg/kg daily, for several days before and after the procedure (strong recommendation; low-quality evidence).
What Is the Treatment for Symptomatic Candida Cystitis?
Recommendations
- For fluconazole-susceptible organisms, oral fluconazole, 200 mg (3 mg/kg) daily for 2 weeks is recommended (strong recommendation; moderate-quality evidence).
- For fluconazole-resistant C. glabrata, AmB deoxycholate, 0.3–0.6 mg/kg daily for 1–7 days OR oral flucytosine, 25 mg/kg 4 times daily for 7–10 days is recommended (strong recommendation; low-quality evidence).
- For C. krusei, AmB deoxycholate, 0.3–0.6 mg/kg daily, for 1–7 days is recommended (strong recommendation; low-quality evidence).
- Removal of an indwelling bladder catheter, if feasible, is strongly recommended (strong recommendation; low-quality evidence).
- AmB deoxycholate bladder irrigation, 50 mg/L sterile water daily for 5 days, may be useful for treatment of cystitis due to fluconazole-resistant species, such as C. glabrata and C. krusei (weak recommendation; low-quality evidence).
What Is the Treatment for Symptomatic Ascending Candida Pyelonephritis?
Recommendations
- For fluconazole-susceptible organisms, oral fluconazole, 200–400 mg (3–6 mg/kg) daily for 2 weeks is recommended (strong recommendation; low-quality evidence).
- For fluconazole-resistant C. glabrata, AmB deoxycholate, 0.3–0.6 mg/kg daily for 1–7 days with or without oral flucytosine, 25 mg/kg 4 times daily, is recommended (strong recommendation; low-quality evidence).
- For fluconazole-resistant C. glabrata, monotherapy with oral flucytosine, 25 mg/kg 4 times daily for 2 weeks, could be considered (weak recommendation; low-quality evidence)
- For C. krusei, AmB deoxycholate, 0.3–0.6 mg/kg daily, for 1–7 days is recommended (strong recommendation; low-quality evidence).
- Elimination of urinary tract obstruction is strongly recommended (strong recommendation; low-quality evidence).
- For patients who have nephrostomy tubes or stents in place, consider removal or replacement, if feasible (weak recommendation; low-quality evidence).
What Is the Treatment for Candida Urinary Tract Infection Associated With Fungus Balls?
Recommendations
- Surgical intervention is strongly recommended in adults (strong recommendation; low-quality evidence).
- Antifungal treatment as noted above for cystitis or pyelonephritis is recommended (strong recommendation; low-quality evidence).
- Irrigation through nephrostomy tubes, if present, with AmB deoxycholate, 25–50 mg in 200–500 mL sterile water, is recommended (strong recommendation; low-quality evidence).
XV. What Is the Treatment for Vulvovaginal Candidiasis?
Recommendations
- For the treatment of uncomplicated Candida vulvovaginitis, topical antifungal agents, with no one agent superior to another, are recommended (strong recommendation; high-quality evidence).
- Alternatively, for the treatment of uncomplicated Candida vulvovaginitis, a single 150-mg oral dose of fluconazole is recommended (strong recommendation; high-quality evidence).
- For severe acute Candida vulvovaginitis, fluconazole, 150 mg, given every 72 hours for a total of 2 or 3 doses, is recommended (strong recommendation; high-quality evidence).
- For C. glabrata vulvovaginitis that is unresponsive to oral azoles, topical intravaginal boric acid, administered in a gelatin capsule, 600 mg daily, for 14 days is an alternative (strong recommendation; low-quality evidence).
- Another alternative agent for C. glabrata infection is nystatin intravaginal suppositories, 100 000 units daily for 14 days (strong recommendation; low-quality evidence).
- A third option for C. glabrata infection is topical 17% flucytosine cream alone or in combination with 3% AmB cream administered daily for 14 days (weak recommendation; low-quality evidence).
- For recurring vulvovaginal candidiasis, 10–14 days of induction therapy with a topical agent or oral fluconazole, followed by fluconazole, 150 mg weekly for 6 months, is recommended (strong recommendation; high-quality evidence).
XVI. What Is the Treatment for Oropharyngeal Candidiasis?
Recommendations
- For mild disease, clotrimazole troches, 10 mg 5 times daily, OR miconazole mucoadhesive buccal 50-mg tablet applied to the mucosal surface over the canine fossa once daily for 7–14 days are recommended (strong recommendation; high-quality evidence).
- Alternatives for mild disease include nystatin suspension (100 000 U/mL) 4–6 mL 4 times daily, OR 1–2 nystatin pastilles (200 000 U each) 4 times daily, for 7–14 days (strong recommendation; moderate-quality evidence).
- For moderate to severe disease, oral fluconazole, 100–200 mg daily, for 7–14 days is recommended (strong recommendation; high-quality evidence).
- For fluconazole-refractory disease, itraconazole solution, 200 mg once daily OR posaconazole suspension, 400 mg twice daily for 3 days then 400 mg daily, for up to 28 days are recommended (strong recommendation; moderate-quality evidence).
- Alternatives for fluconazole-refractory disease include voriconazole, 200 mg twice daily, OR AmB deoxycholate oral suspension, 100 mg/mL 4 times daily (strong recommendation; moderate-quality evidence).
- Intravenous echinocandin (caspofungin: 70-mg loading dose, then 50 mg daily; micafungin: 100 mg daily; or anidulafungin: 200-mg loading dose, then 100 mg daily) OR intravenous AmB deoxycholate, 0.3 mg/kg daily, are other alternatives for refractory disease (weak recommendation; moderate-quality evidence).
- Chronic suppressive therapy is usually unnecessary. If required for patients who have recurrent infection, fluconazole, 100 mg 3 times weekly, is recommended (strong recommendation; high-quality evidence).
- For HIV-infected patients, antiretroviral therapy is strongly recommended to reduce the incidence of recurrent infections (strong recommendation; high-quality evidence).
- For denture-related candidiasis, disinfection of the denture, in addition to antifungal therapy is recommended (strong recommendation; moderate-quality evidence).
XVII. What Is the Treatment for Esophageal Candidiasis?
Recommendations
- Systemic antifungal therapy is always required. A diagnostic trial of antifungal therapy is appropriate before performing an endoscopic examination (strong recommendation; high-quality evidence).
- Oral fluconazole, 200–400 mg (3–6 mg/kg) daily, for 14–21 days is recommended (strong recommendation; high-quality evidence).
- For patients who cannot tolerate oral therapy, intravenous fluconazole, 400 mg (6 mg/kg) daily, OR an echinocandin (micafungin, 150 mg daily, caspofungin, 70-mg loading dose, then 50 mg daily, or anidulafungin, 200 mg daily) is recommended (strong recommendation; high-quality evidence).
- A less preferred alternative for those who cannot tolerate oral therapy is AmB deoxycholate, 0.3–0.7 mg/kg daily (strong recommendation; moderate-quality evidence).
- Consider de-escalating to oral therapy with fluconazole 200–400 mg (3–6 mg/kg) daily once the patient is able to tolerate oral intake (strong recommendation; moderate-quality evidence).
- For fluconazole-refractory disease, itraconazole solution, 200 mg daily, OR voriconazole, 200 mg (3 mg/kg) twice daily either intravenous or oral, for 14–21 days is recommended (strong recommendation; high-quality evidence).
- Alternatives for fluconazole-refractory disease include an echinocandin (micafungin: 150 mg daily; caspofungin: 70-mg loading dose, then 50 mg daily; or anidulafungin: 200 mg daily) for 14–21 days, OR AmB deoxycholate, 0.3–0.7 mg/kg daily, for 21 days (strong recommendation; high-quality evidence)
- Posaconazole suspension, 400 mg twice daily, or extended-release tablets, 300 mg once daily, could be considered for fluconazole-refractory disease (weak recommendation; low-quality evidence).
- For patients who have recurrent esophagitis, chronic suppressive therapy with fluconazole, 100–200 mg 3 times weekly, is recommended (strong recommendation; high-quality evidence).
- For HIV-infected patients, antiretroviral therapy is strongly recommended to reduce the incidence of recurrent infections (strong recommendation; high-quality evidence).
Risk Factors
Patients with the following conditions, treatments or situations are at increased risk for invasive candidiasis.[rx][rx][rx]
- Critical illness
- Long-term intensive care unit stay
- Abdominal surgery (aggravated by anastomotic leakage or repeat laparotomies)
- Immunosuppressive diseases
- Acute necrotizing pancreatitis
- Malignant hematologic disease
- Solid-organ transplantation
- Hematopoietic stem cell transplantation
- Solid-organ tumors
- Neonates (especially low birth weight and preterm infants)
- Broad-spectrum antibiotic treatment
- Central venous catheter
- Internal prosthetic device
- Total parenteral nutrition
- Hemodialysis
- Glucocorticoid use
- Chemotherapy
- Noninvasive Candida colonization (particularly if multifocal)
Transmission
Invasive candidiasis is a nosocomial infection with the majority of cases associated with hospital stays.[rx]
Prevention
These measures may help reduce your risk of developing candida infections
- Rinse your mouth – If you need to use a corticosteroid inhaler, be sure to rinse your mouth with water or brush your teeth after taking your medication.
- Brush your teeth at least twice a day and floss daily – or as often as your dentist recommends.
- Check your dentures – Remove your dentures at night. Make sure dentures fit properly and don’t cause irritation. Clean your dentures daily. Ask your dentist for the best way to clean your type of dentures.
- See your dentist regularly – especially if you have diabetes or wear dentures. Ask your dentist how often you need to be seen.
- Watch what you eat – Try limiting the amount of sugar-containing foods you eat. These may encourage the growth of candida.
- Maintain good blood sugar control if you have diabetes – Well-controlled blood sugar can reduce the amount of sugar in your saliva, discouraging the growth of candida.
- Treat a vaginal yeast infection – as soon as possible.
- Treat dry mouth – Ask your doctor about ways to avoid or treat your dry mouth.
- Avoid douching
- Do not use feminine deodorant or deodorant pads or tampons
- Wear underwear made from cotton or other natural fibers
- Wear loose fitting pants or skirts
- Wash underwear at a high temperature
- Avoid tight underwear and pantyhose
- Eat a healthy, varied diet
- Promptly change wet clothing, for example bathing suits
- Avoid hot tubs and hot baths
Home Remedies
Some home remedies include
- Gentian violet – This is a dye made from coal tar. A person can apply it directly by swabbing it over thrush in the mouth, but they should not swallow it.
- Probiotic-rich foods – Foods that contain probiotics, such as yogurt or cottage cheese, may help the body recover. These foods contain healthful bacteria that may help prevent the growth of thrush.
- Probiotic supplements – Similarly to probiotic-rich foods, these supplements can help the body maintain its healthy bacteria. This may prevent a future thrush infection.
References
