Extramedullary Plasmacytoma is a rare tumor and accounts for 3-5% of all plasma cell neoplasms. Approximately 80% of them occur in the upper aerodigestive tract. Extramedullary plasmacytoma chiefly affects adults older than 65-years-old and there is a male preponderance of 3:1[rx].
Many cases of sinonasal plasmacytoma have been reported to date [rx,rx,rx,rx–rx], few of which involved the maxillary sinus[rx,rx–rx]. Sinonasal plasmacytomas cause different symptoms depending on the sites of origins and the areas of involvement. Their usual symptoms are airway obstruction, nasal discharge, and epistaxis. They can also lead to facial pain and swelling, proptosis, visual impairment, and trismus Solitary extramedullary plasmacytoma shows nonspecific CT and MRI imaging features; however features like a bulky soft tissue mass or infiltrative lesion may suggest its diagnosis. The tumor does not usually become disseminated but may be locally aggressive and destructive to the adjacent structures [rx].
Plasmacytomas originate from the monoclonal malignant transformation of plasma cells. These tumors are a type of B-cell non-Hodgkin lymphoma and encompass a group of neoplasms at different stages of maturity, including multiple myeloma (MM) and solitary plasmacytomas. The latter can be classified into two clinical subsets: solitary plasmacytoma of bone (SPB) and extramedullary plasmacytoma (EMP) [rx,rx]. Although SPB and EMP originate from the same cell type and are initially restricted to a single area, the former tends to evolve into MM more frequently than the latter, and for this reason, the two diseases are often considered different pathologic entities [rx,rx].
Causes Of Extramedullary Plasmacytoma
Different environmental, infectious, and genetic factors have been identified, which predispose to Extramedullary Plasmacytoma.
- Occupational exposure – herbicides, pesticides
- Infectious organisms – These include Helicobacter pylori (MALT lymphoma), Borrelia burgdorferi, Chlamydia psittaci, Campylobacter jejuni, human T- cell lymphotropic virus (adult T- cell leukemia/lymphoma), hepatitis C ( lymphoplasmacytic lymphoma, diffuse large B-cell lymphoma and marginal zone lymphoma), human herpesvirus 8 (primary effusion lymphoma and Castleman disease). Chronic stimulation of lymphoid tissue also increases the risk of lymphoma development. Persistent infection with viruses like Epstein Barr virus and cytomegalovirus also predisposes to the development of lymphoma.[rx][rx]
- Immunodeficiency – HIV infection, transplant recipients, and those with genetic immunodeficiency disorders (severe combined immunodeficiency and common variable immunodeficiency).[rx]
- Drugs – Tumour necrosis factor-alpha inhibitors are associated in particular with T- cell lymphoma. Chronic immunosuppression in post-transplant patients (both solid organ transplant and bone marrow transplant recipients) increases the risk of lymphoma.
- Autoimmune diseases – Inflammatory bowel disease (enteropathy associated lymphoma), rheumatoid arthritis and, Sjögren’s syndrome (diffuse large B-cell lymphoma)
- Geographic location – Extranodal NK/T- cell lymphoma incidence is high in Southern Asia and some parts of Latin America.[rx][rx]
Symptoms of Extramedullary Plasmacytoma
- Often presents with bone pain, lytic bone lesions (thoracic vertebrae most common, also ribs, skull, pelvis, femur); spinal cord compression or peripheral neuropathy are less common presenting symptoms
- Renal failure elevated creatinine hyperuricemia (renal tubular reabsorption of light chain results in damage); hypercalcemia; hypoalbuminemia; hyperviscosity in ~7% (usually IgA or IgG3)
- Recurrent infections due to impaired humoral immunity (immunoglobulin production, often < 50% normal) including Streptococcus pneumonia, Staphylococcus aureus, E. coli
- Anemia (bone marrow infiltration often in areas of most active hematopoiesis and renal failure causing loss of erythropoietin)
- Bone marrow or extramedullary involvement or lytic bone lesions generally associated with advanced disease
- Fun fact: Bence-Jones proteins were the first tumor marker
- References: Approaches to diagnosis, assessment of disease severity and treatment of AL amyloidosis (Clin J Am Soc Nephrol 2006;1:1331), Clinicopathologic data reviewed for 15 cases to delineate the pathology of immunoglobulin M producing multiple myeloma (Am J Clin Pathol 2013;140:519), Workshop focused on salient diagnostic, clinical and genetic features of plasma cell myeloma (Am J Clin Pathol 2011;136:168)
- Asymptomatic (smoldering): more likely to progress to symptomatic myeloma than monoclonal gammopathy of uncertain significance (MGUS); both show gammopathy without symptoms
- Risk of progression 10% per year for the first 5 years
- Lower risk if no progression in the first 5 years after diagnosis (N Engl J Med 2007;356:2582)
- Nonsecretory myeloma ( < 5% of cases): SPE / IFE negative, 85% with impaired secretion and have cytoplasmic Ig by IHC
- 15% nonproducers, serum-free light chain may still be detected
- Lower incidence of renal insufficiency, hypercalcemia, and depression of normal IgG
- Plasma cell leukemia: aggressive with short survival
- > 2 x 109/L or 20% of the leukocyte count on differential are monoclonal plasma cells
- May be primary plasma cell leukemia ( < 5% of myeloma) or represent a late-stage transformation (secondary)
- Typically lack CD56, more frequent abnormal karyotype
- Bone pain and osteolytic lesions less common
- Myeloma cells have the ability to survive and grow outside of the marrow
- Typically associated with extramedullary lesions (e.g. skin, pleural effusions, lymphadenopathy, and organomegaly)
Diagnosis of Extramedullary Plasmacytoma
Diagnosis is based on the detection of the plasma cell tumor in an extramedullary site in the absence of bone marrow plasma cell infiltration, bone lytic lesions, and other signs of multiple myeloma. Extramedullary plasmacytoma must be distinguished from reactive plasma cell lesions and lymphoma. It should be demonstrated that the infiltration consists entirely of plasma cells and that there is no B cell component. In this regard CD138, MUMI/IRF4, CD20, and PAX5 are the most useful markers. Monoclonality and / or an aberrant plasma cell phenotype should be demonstrated with useful markers like CD19, CD56, CD27, CD117, and cyclin D1.
The diagnostic criteria of extramedullary plasmacytoma include:
- Extramedullary tumour of clonal plasma cells
- No M-protein in serum and/or urine
- Normal bone marrow
- Normal skeletal survey
- No related organ or tissue impairment
The following investigations should be performed in all patients diagnosed with extramedullary plasmacytoma:
- Full blood count
- Biochemical screen including electrolytes and corrected calcium
- Serum immunoglobulin levels
- Serum and urine protein electrophoresis and immunofixation
- Serum-free light chain assay
- Full skeletal survey, including standard x-rays of the skeleton including the lateral and anteroposterior cervical, thoracic and lumbar spine, skull, chest, pelvis, humeri, and femora
- MRI of spine and pelvis (or skeletal survey by MRI where this facility exists)
- Bone marrow aspirate and trephine [rx].
The endonasal biopsy of the mass, in this case, had been performed 1.5 years ago and histopathological examination had revealed a small round blue cell tumor with cells positive for the plasma cell marker CD138. Initial investigations exhibited no evidence of anemia, hypercalcemia, or renal involvement. Bence Jones protein was absent in the urine. Bone marrow examination detected no abnormality and serum protein electrophoresis showed no M component. Serum IgG, IgM, and IgA levels were normal. Skeletal survey was done and showed no focal bone disease. In addition, spinal MRI was normal.
The division into these categories will guide the plan for therapy:
- Symptomatic (active) myeloma (Swerdlow: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue, Fourth Edition, 2008):
-
- M protein in serum or urine (usually > 30 g/L of IgG or > 25 g/L IgA or > 1 g/24 hr of urine light chain); no level included in criteria
- Clonal plasma cells in bone marrow or plasmacytoma (usually > 10%, not required)
- Related organ or tissue impairment (CRAB = hypercalcemia, renal failure/insufficiency, anemia, lytic bone lesions)
- Revised NCCN guidelines (J Natl Compr Canc Netw 2016;14:389) diagnostic criteria differ slightly from the WHO 2008 monograph with diagnostic criteria continuing to evolve:
- Clonal bone marrow plasma cells ≥ 10% or biopsy-proven bony or extramedullary plasmacytoma
- And at least one myeloma defining event:
- Calcium > 1 mg/dL above upper limit of normal or > 11 mg/dL
- Creatinine > 2 mg/dL or creatinine clearance < 40 ml/min
- Hemoglobin < 10 g/dL or > 2 g/dL below lower limit of normal
- One or more osteolytic bone lesions on the skeletal radiograph, CT or PET / CT
- Clonal bone marrow plasma cells ≥ 60%
- Involved / uninvolved light chain ratio ≥ 100
- > 1 focal lesions on MRI studies > 5 mm
- Asymptomatic (smoldering) myeloma (J Natl Compr Canc Netw 2016;14:389):
- M protein in serum at ≥ 3 g/dL when IgG or IgA
- Or
- Bence-Jones protein ≥ 500 mg/24h
- 10 – 60% clonal plasma cells in bone marrow
- And
- No related tissue damage/myeloma defining event or amyloidosis; if bone survey negative, the bone disease should be assessed with whole-body MRI or PET / CT
-
- 97% have an M protein in serum or urine, 3% are nonsecretory
- IgG (50%), IgA (20%), light chain (20%), others < 10% (IgD, IgE, IgM and biclonal)
- Serum protein electrophoresis (SPEP): serum proteins normally separate into 5 major fractions based on electric charge and size
- Albumin
- Alpha1 globulins
- Alpha2 globulins
- Beta1 and beta2 globulins
- Gamma globulins
- Gamma globulins include polyclonal antibodies and light chains, with a normal gamma zone appearing as asymmetrical smear, but there is a peak in myeloma.
- Urine protein electrophoresis (UPEP): monoclonal light chains in urine = Bence-Jones protein
- Immunofixation electrophoresis (IFE): used to characterize the M spike, by reacting with specific antisera to heavy chains IgG, IgA, IgM, IgD, IgE, and kappa and lambda light chains.
- Serum-free light chain assay (SFLCA) (Freelite): more sensitive for monitoring light chain disease and nonsecretory myeloma.
| Blood |
| Complete blood count with differential, peripheral blood smear |
| BUN, creatinine, liver enzymes, bilirubin, alkaline phosphatase, total protein, CRP |
| Tumor lysis parameters (LDH, uric acid, calcium, phosphate, potassium) |
| β2-microglobulin and albumin |
| Serum protein electrophoresis, immunofixation, serum-free light chain analysis |
| Immunophenotyping |
| Urine |
| 24-hour urine collection for electrophoresis and immunofixation |
| 24-hour urine for total protein |
| BM |
| Biopsy for histology |
| Aspirate for: |
| Morphology |
| Immunophenotyping |
| Cytogenetic analysis by FISH* focused on del(17p13), del(13q), del(1p21), ample(1q21), t(11;14), t(4;14), and t(14;16) |
| Radiographic skeletal survey, including skull, pelvis, vertebral column, and long bones |
| Additional investigations, which may be useful under certain circumstances |
| Lumbar puncture (cell counts, chemistry, cytology, immunophenotyping): suspicion of leptomeningeal involvement |
| MRI: evaluation of cord compression or painful area of the skeleton (suspicion of soft tissue plasmacytomas arising from bone) |
| CT or 18F-FDG-PET/CT: suspicion of extramedullary plasmacytomas |
| Survey for evaluation of AL amyloidosis |
| Bleeding time, APTT, PT |
| Cryoglobulins, cold agglutinins |
| Serum viscosity, fundoscopy: symptoms of hyperviscosity |
| HLA typing: in case allo-SCT is considered |
- Physical exam and health history – An exam of the body to check general signs of health, including checking for signs of disease, such as lumps or anything else that seems unusual. A history of the patient’s health habits and past illnesses and treatments will also be taken.
- Blood and urine immunoglobulin studies – A procedure in which a blood or urine sample is checked to measure the amounts of certain antibodies (immunoglobulins). For multiple myeloma, beta-2-microglobulin, M protein, free light chains, and other proteins made by the myeloma cells are measured. A higher-than-normal amount of these substances can be a sign of disease.
- Urine Tests – The proteins that myeloma cells make don’t just show up in your blood. They can appear in your urine as well, which gives doctors another way to diagnose the disease. Plus, multiple myeloma can damage your kidneys, so your doctor will want to check your pee for any sign they aren’t working properly. Some of the tests require you to collect urine over a 24-hour period, not give just one sample.
- Bone marrow aspiration and biopsy – The removal of bone marrow, blood, and a small piece of bone by inserting a hollow needle into the hipbone or breastbone. A pathologist views the bone marrow, blood, and bone under a microscope to look for abnormal cells. The following tests may be done on the sample of tissue removed during the bone marrow aspiration and biopsy:
- Cytogenetic analysis – A laboratory test in which the chromosomes of cells in a sample of bone marrow are counted and checked for any changes, such as broken, missing, rearranged, or extra chromosomes. Changes in certain chromosomes may be a sign of cancer. Cytogenetic analysis is used to help diagnose cancer, plan treatment, or find out how well treatment is working.
- FISH (fluorescence in situ hybridization) – A laboratory test used to look at and count genes or chromosomes in cells and tissues. Pieces of DNA that contain fluorescent dyes are made in the laboratory and added to a sample of a patient’s cells or tissues. When these dyed pieces of DNA attach to certain genes or areas of chromosomes in the sample, they light up when viewed under a fluorescent microscope. The FISH test is used to help diagnose cancer and help plan treatment.
- Flow cytometry – A laboratory test that measures the number of cells in a sample, the percentage of live cells in a sample, and certain characteristics of the cells, such as size, shape, and the presence of tumor (or other) markers on the cell surface. The cells from a sample of a patient’s bone marrow are stained with a fluorescent dye, placed in a fluid, and then passed one at a time through a beam of light. The test results are based on how the cells that were stained with the fluorescent dye react to the beam of light. This test is used to help diagnose and manage certain types of cancers, such as leukemia and lymphoma.
- Skeletal bone survey – In a skeletal bone survey, x-rays of all the bones in the body are taken. The x-rays are used to find areas where the bone is damaged. An x-ray is a type of energy beam that can go through the body and onto film, making a picture of areas inside the body.
- Complete blood count (CBC) with differential – A procedure in which a sample of blood is drawn and checked for the following:
- The number of red blood cells and platelets.
- The number and type of white blood cells.
- The amount of hemoglobin (the protein that carries oxygen) in the red blood cells.
- The portion of the blood sample made up of red blood cells.
- Blood chemistry studies – Complete blood count revealed leukocyte of 7,600/μL, granulocyte 5,200/μL, hemoglobin 14.1 g/dL, hematocrit 41.4%, and platelet 327.000/μL. Other initial laboratory tests were as follows: blood urea nitrogen 13 mg/dL; serum creatinine 0.9 mg/dL; sodium 139 mmol/L; potassium K: 3.8 mmol/L; calcium 8.7 mg/dL; phosphorus 3.1 mg/dL; alkaline phosphatase 2223 U/L; aspartate transaminase 190 U/L; alanine transaminase 333 U/L; lactate dehydrogenase 612 U/L; total bilirubin 8.1 mg/dL; direct bilirubin 6.5 mg/dL; total protein 8.2 g/dL; albumin: 3.8 g/dL; erythrocyte sedimentation rate 61 mm/h. Serum protein electrophoresis revealed an M-spike of 2.14 g/dL in gamma globulin region. Urinalysis was insignificant except for bilirubinuria.
- Twenty-four-hour urine test – A test in which urine is collected for 24 hours to measure the amounts of certain substances. An unusual (higher or lower than normal) amount of a substance can be a sign of disease in the organ or tissue that makes it. A higher than normal amount of protein may be a sign of multiple myeloma.
- MRI (magnetic resonance imaging) – A procedure that uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the body. This procedure is also called nuclear magnetic resonance imaging (NMRI). An MRI of the spine and pelvis may be used to find areas where the bone is damaged.
- PET scan (positron emission tomography scan) – A procedure to find malignant tumor cells in the body. A small amount of radioactive glucose (sugar) is injected into a vein. The PET scanner rotates around the body and makes a picture of where glucose is being used in the body. Malignant tumor cells show up brighter in the picture because they are more active and take up more glucose than normal cells do.
- PET-CT scan – A procedure that combines the pictures from a positron emission tomography (PET) scan and a computed tomography (CT) scan. The PET and CT scans are done at the same time with the same machine. The combined scans give more detailed pictures of areas inside the body, such as the spine, than either scan gives by itself.
- Molecular Tests – These highly detailed looks at your bone marrow or tumor cells can identify chromosomes, genes, proteins, and other things that are unique to your cancer. The names of some of these tests are cytogenetics and fluorescent in situ hybridization (FISH). You and your doctors can use the information from these tests to decide on your treatment plan.
- Bone Marrow Biopsy – Multiple myeloma starts in bone marrow, the spongy tissue inside some bones. To test it, the doctor uses medicine to numb the area near your pelvis, then they take a sample of the liquid inside your bone marrow using a needle that goes into your pelvic bone. They also remove a sliver of bone and marrow. Doctors will check the samples to see how your cells look and whether you have too many plasma cells, a sign of multiple myeloma.
- Conventional radiography of the skeleton and computed tomography – Conventional radiography of the skeleton (skeletal survey) has been recommended for the initial assessment of bone lesions for decades. SBP preferentially replaces the trabecular bone, while the cortical bone is partly conserved or even sclerotic [rx]. In two thirds of the cases, the radiographic appearance is characteristic with a mixed, predominantly lytic pattern. Less commonly, SBP has a multicystic appearance. Similar to in MM patients, skeletal surveys are not sensitive enough to detect early lytic bone lesions which are only visible when more than 30% of cortical bone is destructed [rx, rx]. Moreover, conventional X-ray imaging does not reveal EMP located in soft tissues. This underlines the need for other imaging techniques for the evaluation of skeletal and extramedullary lesions, i.e., computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)/CT. In the context of EMP, CT may be helpful for an adequate loco-regional staging as regional lymph node recurrences occur in 7% of EMP cases. CT can also be used to identify spinal cord and/or nerve root compression when MRI is unavailable. Importantly, compression due to a soft tissue mass may be missed on CT scans without contrast injection [rx]. Whole-body (WB)-CT provides high-resolution images of cortical and trabecular bone with a fast scanning time and it is able to detect small (< 5 mm) lytic bone lesions [rx–rx]. Clinical studies addressing the use of WB-CT in comparison with other imaging techniques for SP are currently lacking. Low-dose WB-CT is currently proposed as the initial imaging technique of choice to detect bone disease in patients with MM [rx].
- Magnetic resonance imaging (MRI) – Although the sensitivity of MRI to detect lytic bone lesions is lower than that of CT, MRI is able to detect soft tissue and BM lesions and is the gold standard to detect spinal cord compression. SBP appears as infiltration with a low T1 and a high T2 signal intensity. Moulopoulos et al. prospectively studied the role of MRI in the staging of 12 patients with SBP [rx]. In order to identify other regions of BM involvement in addition to the primary SBP lesion, patients underwent spinal MRI. Additional foci of marrow replacement were found in one-third of patients, and these patients showed persistent elevated serum monoclonal protein levels after radiotherapy. Conversely, patients without additional BM lesions displayed a significant reduction or disappearance of serum monoclonal protein after radiotherapy [rx]. Liebross et al. confirmed these results in a second study in which 57 patients with SBP were staged with either conventional radiography alone or in combination with MRI prior to radiotherapy [rx]. Among 23 patients with thoracolumbar spine disease, 7 of 8 patients, who had a solitary lesion by plain radiographs alone, developed MM in comparison with 1 of 7 patients who also had only one lesion by MRI. Together, these prospective studies underline the importance of precise staging. Consequently, excluding additional lesions is mandatory for the diagnosis of SP as per IMWG criteria [rx]. The use of diffusion-weighted MRI, a new highly sensitive technique to detect and monitor tumor lesions in the BM and soft tissues, has not been reported in the management of SP.
- Positron emission tomography/computed tomography (PET/CT) – While 18F-FDG (fluorine-18-fludeoxyglucose) PET/CT is extensively studied in MM, only a few small-scale studies have addressed its role in SP [rx]. 18F-FDG PET or 18F-FDG PET/CT may show additional lesions and have therapeutic implications in 33–55% of patients with presumed SBP as patients with a normal PET/CT did not develop MM [rx–rx]. Salaun et al. reported that 18F-FDG PET/CT is superior to MRI for the diagnosis and follow-up of plasmacytoma patients, although it should be noted that this study was performed in the context of extramedullary spread during MM [rx]. At diagnosis, the sensitivity and specificity of PET/CT was higher than that of MRI of the spine and pelvis, because PET/CT was able to detect plasmacytoma lesions with a larger scope compared to MRI, i.e., in soft tissues, skull, ribs, and limbs. Also, the specificity of PET/CT was 99% to evaluate treatment responses, compared to 89% for MRI. A second study evaluated PET/CT and axial MRI at diagnosis and follow-up in 43 patients with either EMP (10 patients) or SBP (33 patients) [rx]. PET/CT at diagnosis identified 10 patients with at least two hypermetabolic lesions. Six of these patients progressed to MM, confirming a diagnostic role for PET/CT in SP. FDG-uptake was recently found to be correlated to the tumor size [rx]: FDG-avid lesions are generally larger (41.4 mm) vs. FDG-negative lesions (23.5 mm) and the statistical risk of progression was higher in these FDG-avid lesions [rx]. Based on these studies, the IMWG recommended PET/CT as part of the initial evaluation of patients with SP [rx].
- Chest computed tomography – showed a mass of 5 × 4 cm in size, destructing the rib of the right chest wall, two nodular lesions of 2 cm and 3.5 cm in size, situated in the subcutaneous fatty layer of right and left chest walls, respectively, and a seemingly benign lymph node of 1.2 cm in the perivascular space of mediastinum. Abdominal magnetic resonance imaging and MR cholangiopancreatography disclosed dilated intrahepatic biliary ducts, gall bladder hydrops with a 6 mm polyp, moderately dilated common bile duct (16 mm), a solid mass, 4.5 cm in diameter, in the pancreatic head, a regularly contoured mass measuring 26 × 18 mm in diameter in the left adrenal gland, a mass of 2 cm in the superior lobe of left kidney, and a mass of 2.8 cm in the inferior splenic pole; in addition, multiple masses varying in size were seen in the abdominal oblique muscle, left pararectal space, right iliac and ischial bones, sacroiliac wing, close proximity to the inferior pole of left kidney, and left perirectal fossa. Bone scintigraphy demonstrated increased activity in the anterolateral aspect of eighth left rib, posterior aspect of seventh right rib, posterior aspects of fourth and sixth left ribs, right scapula, left the tibiotalar area, distal diaphysis of left femur, and both of iliac wings. Tru-cut biopsies were performed from skin lesions and mass located in the pancreatic head. Neoplastic cell infiltration intermingled with areas of fibrosis and subtle necrosis, originating in the papillary dermis and extending down into subcutaneous tissue corresponding to the lower margin of the biopsy sample, was seen on skin biopsy. Morphologically, neoplastic cells splayed between collagen bundles in the dermis appeared to have plasmacytoid-plasmablastic differentiation. Immunohistochemical staining for CD38 and kappa and lambda light chains was carried out. It revealed that neoplastic cells showed a monotypic light chain restriction with positive kappa light chains. Tru-cut biopsy from the pancreatic mass was performed and histopathological and immunohistochemical findings were similar to those encountered in skin mass biopsy: neoplastic cell infiltrates interposed between areas of fibrosis and subtle necrosis, plasmacytoid-plasmablastic differentiation, and kappa light chain positive plasma cell dominance. A final diagnosis of multiple myeloma complicated with extramedullary plasmacytomas involving the pancreas, suprarenal gland, kidney, skin, lung, liver, spleen, and lymph nodes was attained.
Treatment of Extramedullary Plasmacytoma
Chemotherapy
In most series, adjuvant chemotherapy (CHT) has no beneficial effect on disease control or prevention of progression to multiple myeloma [rx, rx]. A survival advantage was stated in a study that compared adjuvant melphalan and prednisone given for three years after RT with “RT alone”. Nevertheless, the study concerned included a small number of patients [rx]. For the patients with tumors larger than 5 cm and high-grade histology, adjuvant CHT may be considered [rx]. CHT may also be considered to unresponded patients to RT. Treatment schedules that are effective against multiple myeloma can be considered for these patients [rx]. Holland et al. showed that CHT delays the progression time of plasmacytoma to MM. Nevertheless, its use did not decrease the conversion rate [rx]. Besides, after progression to MM, the patients, who received CHT, had the same survival time as those patients who did not receive CHT [rx]. Furthermore, it is suggested that early exposure to CHT may speed up the progression of resistant subclones and, therefore, limit later therapeutic options, when they may be more beneficial [rx]. In addition, in one series, secondary leukemia developed in 4 of 7 patients with SBP who received adjuvant melphalan-based CHT after RT had been completed [rx].
Conventional chemotherapy
The prognosis of pPCL after conventional chemotherapy without novel agents is poor, with median OS of ∼ 7 months.[rx–rx] There appears to be limited benefit in terms of survival for multiagent conventional chemotherapy, such as vincristine, adriamycin, and dexamethasone (VAD)-based regimens, compared with regimens containing only an alkylating agent plus a corticosteroid.[rx–rx]
Novel agents
The introduction of immunomodulatory drugs and proteasome inhibitors has significantly improved the survival of MM patients.[rx,rx] Increasing evidence suggests that these agents also improve the outcome of pPCL, but the benefit may be less pronounced compared with classic MM. A retrospective analysis performed by the Intergroup Francophone du Myélome showed that pPCL patients treated with novel agents had a survival of 15 months compared with 8 months for patients who did not receive novel agents as part of their treatment.[rx] In addition, a retrospective analysis performed by GIMEMA showed improved survival for those patients who received bortezomib and/or thalidomide at any stage of their treatment.[rx] In contrast, a SEER database analysis failed to show enhanced survival of pPCL patients in the period 1973-2004, but information on treatment changes over time were lacking in this study.[rx] There remains a limited number of prospective studies evaluating novel agents in pPCL, with several retrospective studies providing additional information on the efficacy of these drugs.
Drug therapy
Corticosteroids are steroids that have antitumor effects in multiple myeloma. Drugs such as prednisone and dexamethasone can boost your immune system, fight inflammation, and work against the myeloma cells in your body. Your doctor might have you take a steroid as part of your treatment plan. You can take these drugs in pill form, or get them as shots into a vein in your arm.
Targeted therapy
Targeted therapy is a treatment that uses drugs or other substances to identify and attack specific cancer cells. Targeted therapy may cause less harm to normal cells than chemotherapy or radiation therapy do. Several types of targeted therapy may be used to treat multiple myeloma and other plasma cell neoplasms. There are different types of targeted therapy:
- Proteasome inhibitor therapy – This treatment blocks the action of proteasomes in cancer cells. A proteasome is a protein that removes other proteins no longer needed by the cell. When the proteins are not removed from the cell, they build up and may cause the cancer cell to die. Bortezomib, carfilzomib, and ixazomib are proteasome inhibitors used in the treatment of multiple myeloma and other plasma cell neoplasms.
- Monoclonal antibody therapy – This treatment uses antibodies made in the laboratory, from a single type of immune system cell. These antibodies can identify substances on cancer cells or normal substances that may help cancer cells grow. The antibodies attach to the substances and kill the cancer cells, block their growth, or keep them from spreading. Monoclonal antibodies are given by infusion. They may be used alone or to carry drugs, toxins, or radioactive material directly to cancer cells. Daratumumab and elotuzumab are monoclonal antibodies used in the treatment of multiple myeloma and other plasma cell neoplasms. Denosumab is a monoclonal antibody used to slow bone loss and reduce bone pain in patients with multiple myeloma.
- Histone deacetylase (HDAC) inhibitor therapy – This treatment blocks enzymes needed for cell division and may stop the growth of cancer cells. Panobinostat is an HDAC inhibitor used in the treatment of multiple myeloma and other plasma cell neoplasms.
- BCL2 inhibitor therapy – This treatment blocks a protein called BCL2. Blocking this protein may help kill cancer cells and may make them more sensitive to anticancer drugs. Venetoclax is a BCL2 inhibitor being studied in the treatment of relapsed or refractory multiple myeloma.
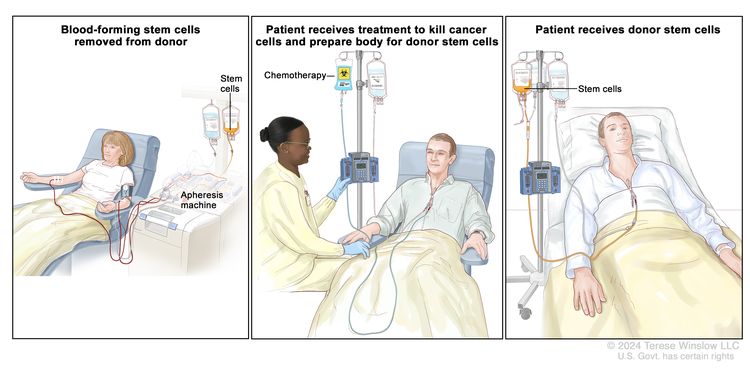
High-dose chemotherapy with stem cell transplant
High doses of chemotherapy are given to kill cancer cells. Healthy cells, including blood-forming cells, are also destroyed by cancer treatment. A stem cell transplant is a treatment to replace the blood-forming cells. Stem cells (immature blood cells) are removed from the blood or bone marrow of the patient (autologous) or a donor (allogeneic) and are frozen and stored. After the patient completes chemotherapy, the stored stem cells are thawed and given back to the patient through an infusion. These reinfused stem cells grow into (and restore) the body’s blood cells.
Immunotherapy
Immunotherapy is a treatment that uses the patient’s immune system to fight cancer. Substances made by the body or made in a laboratory are used to boost, direct, or restore the body’s natural defenses against cancer. This type of cancer treatment is also called biotherapy or biologic therapy.
- Immunomodulator therapy – Thalidomide, lenalidomide, and pomalidomide are immunomodulators used to treat multiple myeloma and other plasma cell neoplasms.
- Interferon – This treatment affects the division of cancer cells and can slow tumor growth.
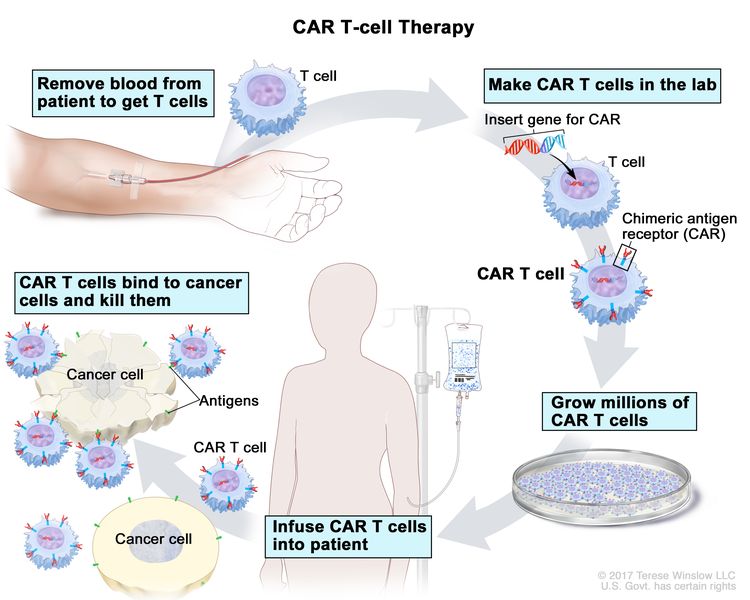
- CAR T-cell therapy – This treatment changes the patient’s T cells (a type of immune system cell) so they will attack certain proteins on the surface of cancer cells. T cells are taken from the patient and special receptors are added to their surface in the laboratory. The changed cells are called chimeric antigen receptor (CAR) T cells. The CAR T cells are grown in the laboratory and given to the patient by infusion. The CAR T cells multiply in the patient’s blood and attack cancer cells. CAR T-cell therapy is being studied in the treatment of multiple myeloma that has recurred (come back).
CAR T-cell therapy – A type of treatment in which a patient’s T cells (a type of immune cell) are changed in the laboratory so they will bind to cancer cells and kill them. Blood from a vein in the patient’s arm flows through a tube to an apheresis machine (not shown), which removes the white blood cells, including the T cells, and sends the rest of the blood back to the patient. Then, the gene for a special receptor called a chimeric antigen receptor (CAR) is inserted into the T cells in the laboratory. Millions of the CAR T cells are grown in the laboratory and then given to the patient by infusion. The CAR T cells are able to bind to an antigen on the cancer cells and kill them.
Radiation therapy
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. External radiation therapy uses a machine outside the body to send radiation toward the area of the body with cancer.
Watchful waiting
Watchful waiting is closely monitoring a patient’s condition without giving any treatment until signs or symptoms appear or change. When you have multiple myeloma, cancer cells crowd out healthy blood cells in your body. Instead of making normal proteins called antibodies, they make particles called M proteins. (The M stands for monoclonal.) To diagnose you, your doctor will take a small amount of blood from a vein in your arm. A lab then checks it for M proteins and another substance — beta-2 microglobulin — that are signs you have multiple myeloma.
Supportive care is given to lessen the problems caused by the disease or its treatment.
This therapy controls problems or side effects caused by the disease or its treatment, and improves quality of life. Supportive care is given to treat problems caused by multiple myeloma and other plasma cell neoplasms.
Supportive care may include the following:
- Plasmapheresis – If the blood becomes thick with extra antibody proteins and interferes with circulation, plasmapheresis is done to remove extra plasma and antibody proteins from the blood. In this procedure blood is removed from the patient and sent through a machine that separates the plasma (the liquid part of the blood) from the blood cells. The patient’s plasma contains the unneeded antibodies and is not returned to the patient. The normal blood cells are returned to the bloodstream along with donated plasma or a plasma replacement. Plasmapheresis does not keep new antibodies from forming.
- High-dose chemotherapy with stem cell transplant – If amyloidosis occurs, treatment may include high-dose chemotherapy followed by stem cell transplant using the patient’s own stem cells.
- Immunotherapy – Immunotherapy with thalidomide, lenalidomide, or pomalidomide is given to treat amyloidosis.
- Targeted therapy – Targeted therapy with proteasome inhibitors is given to decrease how much immunoglobulin M is in the blood and treat amyloidosis. Targeted therapy with a monoclonal antibody is given to slow bone loss and reduce bone pain.
- Radiation therapy – Radiation therapy is given for bone lesions of the spine.
- Chemotherapy – Chemotherapy is given to reduce back pain from osteoporosis or compression fractures of the spine.
- Bisphosphonate therapy – Bisphosphonate therapy is given to slow bone loss and reduce bone pain. See the following PDQ summaries for more information on bisphosphonates and problems related to their use:
- Cancer Pain
- Oral Complications of Chemotherapy and Head/Neck Radiation
References