Mechanisms of Hormone Action/Hormones are the messenger molecules of the endocrine system. Endocrine hormones travel throughout the body in the blood. However, each hormone affects only certain cells, called target cells. A target cell is the type of cell on which a hormone has an effect. A target cell is affected by a particular hormone because it has receptor proteins that are specific to that hormone. A hormone travels through the bloodstream until it finds a target cell with a matching receptor it can bind to. When the hormone binds to a receptor, it causes a change within the cell. Exactly how this works depends on whether the hormone is a steroid hormone or a non-steroid hormone.
Mechanisms of Hormone Action
Protein and peptide hormones, catecholamines like epinephrine, and eicosanoids such as prostaglandins find their receptors decorating the plasma membrane of target cells.
The binding of the hormone to the receptor initiates a series of events which leads to the generation of so-called second messengers within the cell (the hormone is the first messenger). The second messengers then trigger a series of molecular interactions that alter the physiologic state of the cell. Another term used to describe this entire process is signal transduction.
Structure of Cell Surface Receptors
Cell surface receptors are integral membrane proteins and, as such, have regions that contribute to three basic domains:
- Extracellular domains: Some of the residues exposed to the outside of the cell interact with and bind the hormone – another term for these regions is the ligand-binding domain.
- Transmembrane domains: Hydrophobic stretches of amino acids are “comfortable” in the lipid bilayer and serve to anchor the receptor in the membrane.
- Cytoplasmic or intracellular domains: Tails or loops of the receptor that are within the cytoplasm react to hormone binding by interacting in some way with other molecules, leading to the generation of second messengers. Cytoplasmic residues of the receptor are thus the effector region of the molecule.
Several distinctive variations in receptor structure have been identified. As depicted below, some receptors are simple, single-pass proteins; many growth factor receptors take this form. Others, such as the receptor for insulin, have more than one subunit. Another class, which includes the beta-adrenergic receptor, is threaded through the membrane seven times.
Receptor molecules are neither isolated by themselves nor fixed in one location of the plasma membrane. In some cases, other integral membrane proteins interact with the receptor to modulate its activity. Some types of receptors cluster together in the membrane after binding hormones. Finally, as elaborated below, the interaction of the hormone-bound receptor with other membrane or cytoplasmic proteins is the key to the generation of second messengers and transduction of the hormonal signal.
Second Messenger Systems
Consider what would happen if, late at night, you noticed a building on fire. Hopefully, you would dial 911 or a similar emergency number. You would inform the dispatcher of the fire, and the dispatcher would, in turn, contact and “activate” a number of firemen. The firefighters would then rapidly go to work pouring water on the fire, setting up roadblocks, and the like. They would also probably activate other “players”, such as police and fire investigators that would come in later to try and determine the cause of the fire. Importantly, once the fire is out (or the building totally destroyed), the firemen go back to the station and to sleep.
The community response to a fire is, at least in some ways, analogous to a second messenger system involved in a hormone’s action. In the scenario described, you are the “first messenger”, the dispatcher is the “receptor”, the firefighters are the “second messengers”.
Currently, four-second messenger systems are recognized in cells, as summarized in the table below. Note that not only do multiple hormones utilize the same second messenger system, but a single hormone can utilize more than one system. Understanding how cells integrate signals from several hormones into a coherent biological response remains a challenge.
| Second Messenger | Examples of Hormones Which Utilize This System |
|---|---|
| Cyclic AMP | Epinephrine and norepinephrine, glucagon, luteinizing hormone, follicle-stimulating hormone, thyroid-stimulating hormone, calcitonin, parathyroid hormone, antidiuretic hormone |
| Protein kinase activity | Insulin, growth hormone, prolactin, oxytocin, erythropoietin, several growth factors |
| Calcium and/or phosphoinositides | Epinephrine and norepinephrine, angiotensin II, antidiuretic hormone, gonadotropin-releasing hormone, thyroid-releasing hormone. |
| Cyclic GMP | Atrial natriuretic hormone, nitric oxide |
In all cases, the seemingly small signal generated by hormone binding its receptor is amplified within the cell into a cascade of actions that changes the cell’s physiologic state. Presented below are two examples of second messenger systems commonly used by hormones. The examples used are of glucagon and insulin, both of which ultimately work through a molecular switch involving protein phosphorylation. Be aware that in both cases, a very complex system is being simplified considerably.
Cyclic AMP Second Messenger Systems
Cyclic adenosine monophosphate (cAMP) is a nucleotide generated from ATP through the action of the enzyme adenylate cyclase. The intracellular concentration of cAMP is increased or decreased by a variety of hormones and such fluctuations affect a variety of cellular processes. One prominent and important effect of elevated concentrations of cAMP is the activation of a cAMP-dependent protein kinase called protein kinase A.
Protein kinase A is nominally in a catalytically inactive state but becomes active when it binds cAMP. Upon activation, protein kinase A phosphorylates a number of other proteins, many of which are themselves enzymes that are either activated or suppressed by being phosphorylated. Such changes in enzymatic activity within the cell clearly alter its state.
Now, let’s put this information together to understand the mechanism of action of a hormone-like glucagon
- Glucagon binds its receptor in the plasma membrane of target cells (e.g. hepatocytes).
- Bound receptor interacts with and, through a set of G proteins, turns on adenylate cyclase, which is also an integral membrane protein.
- Activated adenylate cyclase begins to convert ATP to cyclic AMP, resulting in an elevated intracellular concentration of cAMP.
- High levels of cAMP in the cytosol make it probable that protein kinase A will be bound by cAMP and therefore catalytically active.
- Active protein kinase A “runs around the cell” adding phosphates to other enzymes, thereby changing their conformation and modulating their catalytic activity – – – abracadabra, the cell has been changed!
- Levels of cAMP decrease due to destruction by cAMP-phosphodiesterase and the inactivation of adenylate cyclase.
In the above example, the hormone’s action was to modify the activity of pre-existing components in the cell. Elevations in cAMP also have important effects on the transcription of certain genes.
Tyrosine Kinase Second Messenger Systems
The receptors for several protein hormones are themselves protein kinases which are switched on by the binding of hormones. The kinase activity associated with such receptors results in the phosphorylation of tyrosine residues on other proteins. Insulin is an example of a hormone whose receptor is a tyrosine kinase.
The hormone binds to domains exposed on the cell’s surface, resulting in a conformational change that activates kinase domains located in the cytoplasmic regions of the receptor. In many cases, the receptor phosphorylates itself as part of the kinase activation process. The activated receptor phosphorylates a variety of intracellular targets, many of which are enzymes that become activated or are inactivated upon phosphorylations was seen with cAMP second messenger systems, activation of receptor tyrosine kinases leads to rapid modulation in a number of target proteins within the cell. Interestingly, some of the targets of receptor kinases are protein phosphatases which, upon activation by receptor tyrosine kinase, become competent to remove phosphates from other proteins and alter their activity. Again, a seemingly small change due to hormone binding is amplified into a multitude of effects within the cell.
In some cases, the binding of the hormone to a surface receptor induces a tyrosine kinase cascade even though the receptor is not itself a tyrosine kinase. The growth hormone receptor is one example of such a system – the interaction of growth hormone with its receptor leads to activation of cytoplasmic tyrosine kinases, with results conceptually similar to that seen with receptor kinases.
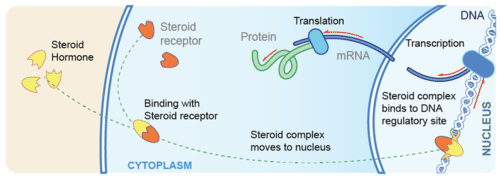
Steroid Hormones
Steroid hormones are made of lipids, such as phospholipids and cholesterol. They are fat-soluble, so they can diffuse across the plasma membrane of target cells and bind with receptors in the cytoplasm of the cell. The steroid hormone and receptor form a complex that moves into the nucleus and influences the expression of genes, essentially acting as a transcription factor. Examples of steroid hormones include cortisol and sex hormones.
Asteroid hormone crosses the plasma membrane of a target cell and binds with a receptor inside the cell.
Non-Steroid Hormones
Non-steroid hormones are made of amino acids. They are not fat-soluble, so they cannot diffuse across the plasma membrane of target cells. Instead, a non-steroid hormone binds to a receptor on the cell membrane. The binding of the hormone triggers an enzyme inside the cell membrane. The enzyme activates another molecule, called the second messenger, which influences processes inside the cell. Most endocrine hormones are non-steroid hormones, including insulin and thyroid hormones.
Direct Gene Activation and the Second-Messenger System
Nuclear receptors function as transcription factors because they can bind to DNA and regulate gene expression.
Key Points
Receptors that can directly influence gene expression are termed nuclear receptors.
Type I nuclear receptors (found in cytosol) are modified to translocate to the nucleus upon hormone binding.Type II nuclear receptors remain in the nucleus where they often create a complex with co-repressor proteins, which are released upon hormone binding.
Secondary messengers relay signals from receptors on the cell surface to the target molecules.
The secondary messenger systems bind hormones to a receptor that causes a cascade of changes that leads to actions.
Key Terms
nuclear receptor: A class of proteins found within cells that are responsible for sensing steroid and thyroid hormones and certain other molecules, as well as influencing gene expression upon activation.
secondary messenger: Molecules that relay signals from receptors on the cell surface to target molecules inside the cell, in the cytoplasm or nucleus.
hormone response element: A short sequence of DNA within the promoter of a gene that is able to bind a specific hormone-receptor complex and therefore regulate gene expression.
Hormones can alter cell activity by binding with a receptor. Receptors can either directly influence gene expression and thus cell activity, or induce a secondary signaling cascade that will in turn influence cell activity.
Direct Gene Activation
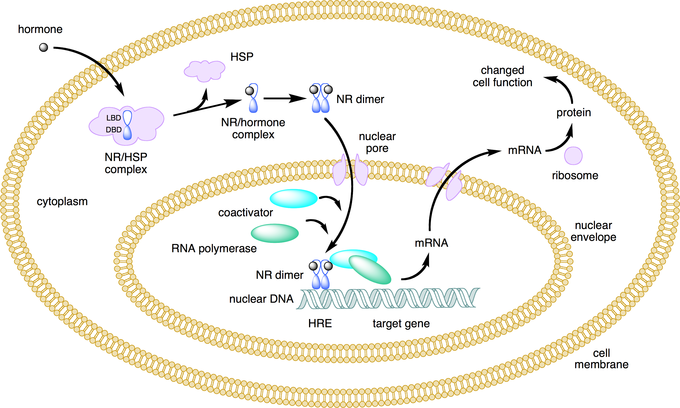
Receptors that can directly influence gene expression are termed nuclear receptors. Located within the cytosol or nucleus, nuclear receptors are the target of steroid and thyroid hormones that are able to pass through the cell membrane. Nuclear receptors can bind directly to DNA to regulate specific gene expressions and are, therefore, classified as transcription factors.
Nuclear receptors can be classified into two broad classes according to their mechanism of action and their sub-cellular distribution in the absence of ligand. Type I nuclear receptors are located in the cytosol. Upon binding to a hormone the receptor and hormone translocate into the nucleus, and bind to specific sequences of DNA known as hormone response elements (HREs).
Type II receptors are retained in the nucleus. In the absence of ligand, type II nuclear receptors often form a complex with co-repressor proteins. Hormone binding to the nuclear receptor results in dissociation of the co-repressor and the recruitment of co-activator proteins.
Lipid soluble hormones directly regulate gene expression: This figure depicts the mechanism of a class I nuclear receptor (NR) that, in the absence of ligand, is located in the cytosol. Hormone binding to the NR triggers translocation to the nucleus, where the NR binds to a specific sequence of DNA known as a hormone response element (HRE).
Secondary Messengers
For lipophobic hormones that cannot pass the cellular membrane, activity is mediated and amplified within a cell by the action of second messenger mechanisms (molecules that relay signals from receptors on the cell surface to target molecules inside the cell in the cytoplasm or nucleus).
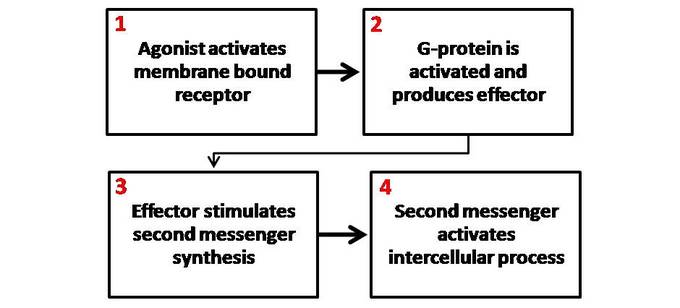
Most hormone receptors are G protein-coupled receptors. Upon hormone binding, the receptor undergoes a conformational change and exposes a binding site for a G-protein. The G-protein is bound to the inner membrane of the cell and consists of three sub-units: alpha, beta, and gamma.
Upon binding to the receptor, it releases a GTP molecule, at which point the alpha sub-unit of the G-protein breaks free from the beta and gamma sub-units and is able to move along the inner membrane until it contacts another membrane-bound protein: the primary effector.
The primary effector then has an action, which creates a signal that can diffuse within the cell. This signal is called the secondary messenger. The secondary messenger may then activate a secondary effector, whose effects depend on the particular secondary messenger system.
Second messenger mechanisms: General schematic of second messenger generation following activation of membrane-bound receptors. 1. The agonist activates the membrane-bound receptor. 2. G-protein is activated and produces an effector. 3. The effector stimulates a second messenger synthesis. 4. The second messenger activates an intercellular process.
Target Cell Specificity
Hormones target a limited number of cells (based on the presence of a specific receptor) as they circulate in the bloodstream.
Key Points
Target cells are cells that are receptive to a secreted hormone.
Target cell activation is
dependent on three factors; the hormone levels in the blood, the receptor levels on the target cell, and hormone–receptor affinity.
Key Terms
target cell: A cell that is receptive to a secreted hormone.
EXAMPLES
An XY fetus will develop along a female pathway if the target cells fail to respond to androgen. This androgen insensitivity occurs when the receptors on the target cells are unable to accept the hormone due to an impairment in receptor shape.
In endocrinology, target cells can refer to the cells where hormones have an effect. Target cells are capable of responding to hormones because they display receptors to which the circulating hormone can bind. In this way, hormones only affect a limited number of cells even though they are transported in the bloodstream throughout the body.
Target cell activation is dependent on three factors:
- The levels of hormones in the blood.
- The relative number of hormone receptors on the target cell.
- The hormone–receptor affinity.
Modulation of these factors can control target cell response. For example, after receptor stimulation, the signaling target cell often sends feedback to the hormone-secreting tissue to down-regulate hormone expression.
Additionally, the target cell can up or down-regulate receptor expression to make it more or less sensitive to the same hormone. Finally, hormone–receptor affinity can be altered by the expression of associated inhibitory or co-activating factors.
In some instances, alterations of receptor structure due to a genetic mutation can lead to a reduction in hormone–receptor affinity, as in the case of androgen insensitivity.
Onset, Duration, and Half-Life of Hormone Activity
A hormone’s half-life and duration of activity are limited and vary from hormone to hormone.
Key Points
The hormone receptors are dynamic structures that vary in number and sensitivity, that depend on the levels of the stimulating hormone.
The blood levels of hormones reflect a balance between secretion and degradation/excretion by the liver and kidneys.
The biological half- life of a hormone is the time it takes for the hormone to lose half of its physiological activity.
The duration of hormone activity refers to the duration of altered cellular behavior triggered by hormone binding.
Key Terms
hormone receptor: A molecule that binds to a specific hormone that triggers alterations in cell activity.
half-life: The time it takes for a substance (drug, radioactive nuclide, or other) to lose half of its pharmacological, physiological, or radiological activity.
EXAMPLES
Vitamin D is a hormone that has a half-life of one to two months. If one obtains vitamin D solely through sun (UVB) exposure during the summer months, serum vitamin D levels will be critically low by late winter. This is one reason why current recommendations are to take vitamin D supplements in order to maintain serum vitamin D levels throughout the year.
The number of hormone molecules available for complex formation is usually the key factor that determines the level at which signal transduction pathways are activated. The number of hormone molecules that are available is determined by the concentration of circulating hormones.
Half-Life
The blood levels of hormones reflect a balance between synthesis/secretion and degradation/excretion. The liver and kidneys are the major organs that degrade hormones with breakdown products excreted in urine and feces.
A hormone’s half-life and duration of activity are limited and vary from hormone to hormone. For instance, the biological half-life of luteinizing hormone is 20 minutes, which is shorter than that of a follicle-stimulating hormone (three to four hours), and of human chorionic gonadotropin (24 hours).
A biological half-life or elimination half-life is the time it takes for a substance such as a hormone or a drug to lose half of its pharmacologic or physiologic activity. In a medical context, the half-life may also describe the time it takes for the blood plasma concentration of a substance to halve (plasma half-life) its steady state.
The relationship between the biological and plasma half-lives of a substance can be complex, due to factors including their accumulation in tissues, active metabolites, and receptor interactions.
Duration
The duration of hormone activity refers to the duration of events that were stimulated by hormone-receptor binding. While typically relatively short and measured in minutes or hours, certain events, such as the onset of puberty, are much longer-lasting.
Hormone levels during the menstrual cycle: This image depicts the levels of certain hormones during the menstrual cycle (B), as they correspond to follicular growth and ovulation (A). 1. Follicle-stimulating hormone 2. Estrogen 3. Luteinizing hormone 4. Progesterone.
Mechanism
Growth Hormone
Growth Hormone has a direct and indirect mechanism of action. The direct effect of growth hormone involves growth hormone directly binding to its receptors on target cells to stimulate a response. The indirect effect is mediated by the action of insulin-like growth factor-1(IGF-1), which is secreted by the liver hepatocytes in response to growth hormone. Insulin-like growth factor-1 binds to its receptor, IGF-1R, on the cellular surface and activates a tyrosine kinase-mediated intracellular signaling pathway that phosphorylates various proteins intracellularly leading to increased anabolism, cellular replication and division, and metabolism.[rx]
Prolactin
Prolactin initiates its effect by binding to the prolactin receptor found on various tissues across the body, including but not limited to mammillary glands, ovaries, skeletal muscle, uterus, and thymus. Upon the binding of prolactin to its receptor, Jannu kinase 2, a tyrosine kinase is activated that furthermore initiates the JAK-STAT pathway.
FSH & LH
Both LH & FSH bind to G protein-coupled receptors. Upon binding to the receptor, adenylyl cyclase, an enzyme is activated, which goes on to produce cyclic-AMP. The intracellular concentrations of cyclic-AMP rise, which further activates a kinase molecule called protein kinase A. Protein kinase A primarily functions to phosphorylate specific intracellular proteins that then subsequently complete the physiological actions of FSH and LH.
Adrenocorticotropic Hormone
ACTH interacts with G protein-coupled receptors found on the extracellular membranes of the zona fasciculata and zona reticularis of the adrenal cortex. cAMP is the secondary messenger system. Activation of the g-couple receptor activates adenylyl cyclase, thus increase cAMP production and subsequent activation of Protein Kinase A.[rx]
Thyroid Stimulating Hormone (TSH)
TSH binds and activates the TSH receptor (TSHR) found on the basolateral surface of thyroid follicle cells. This binding site is a G-protein coupled receptor (GPCR), which couples to both Gs and Gq G-proteins, and hence activating both the cAMP pathway (via Gsa) and the phosphoinositol/calcium (IP/Ca2+; via Gq) second messenger signaling cascades. The Gs pathway activates iodide uptake, increases thyroid hormone production, and enhances gland growth and differentiation. The Gq pathway is rate-limiting for hormone production by stimulating iodide organification.[rx]
Vasopressin
Two regulating receptors, the subfornical organ and the organum vasculum in the hypothalamus, since water deprivation and signal for ADH secretion.[rx] A small concentration of vasopressin is sufficient to generate water conservation in the renal tubules. The renal tubules are divided into the proximal, descending, ascending, distal regions, and the collecting duct. The most ADH-dependent segment of the renal tubule is the collecting duct, which has ADH receptors on its basolateral side for ADH to bind and stimulate the Gs protein. The Gs protein stimulates adenylyl cyclase, which further converts ATP into cAMP. High levels of cAMP cause the phosphorylation of protein kinase A, subsequently opening water channels known as aquaporins to allow passage of water from the luminal side to the basolateral side.
Oxytocin
Oxytocin binds to its extracellular receptor present in the myometrium of the uterus, which then activates the Gq protein further leading to activation of phospholipase C. The phospholipase C functions to break down the phosphoinositol diphosphate into two components, Inositol triphosphate (IP3) which will release calcium from the sarcoplasmic reticulum and diacylglycerol (DAG) which will activate protein kinase C. The protein kinase C phosphorylates proteins specifically on the cell membrane to allow calcium entry from the extracellular space. The increased intracellular calcium generates enough energy to cause the contraction of the uterus.
During lactation, when the newborn suckles, it transmits signals to the central nervous system to release oxytocin, a process known as the “milk letdown reflex.” The oxytocin binds to the breast myoepithelial cell receptors and initiates the same Gq cascade similar to uterine contraction, and ejects milk into the baby’s oral cavity.
References