Lipids are compounds that are insoluble in water but are soluble in organic solvents such as ether and chloroform. Lipids that are important to our discussion include fats and oils (triglycerides or triacylglycerols), fatty acids, phospholipids, and cholesterol. Fats and oils are esters of glycerol and three fatty acids. They are important in the diet as energy sources and as sources of essential fatty acids and fat-soluble vitamins, which tend to associate with fats. They also contribute satiety, flavor, and palatability to the diet.
Lipid Molecules
Fats and oils, which may be saturated or unsaturated, can be unhealthy but also serve important functions for plants and animals.
Key Points
Fats provide energy, insulation, and storage of fatty acids for many organisms.
Fats may be saturated (having single bonds) or unsaturated (having double bonds).
Unsaturated fats may be cis (hydrogens in the same plane) or trans (hydrogens in two different planes).
Olive oil, a monounsaturated fat, has a single double bond whereas canola oil, a polyunsaturated fat, has more than one double bond.
Omega-3 fatty acids and omega-6 fatty acids are essential for human biological processes, but they must be ingested in the diet because they cannot be synthesized.
Key Terms
- hydrogenation: The chemical reaction of hydrogen with another substance, especially with an unsaturated organic compound, and usually under the influence of temperature, pressure and catalysts.
- ester: Compound most often formed by the condensation of an alcohol and an acid, by removing water. It contains the functional group carbon-oxygen double bond joined via carbon to another oxygen atom.
- carboxyl: A univalent functional group consisting of a carbonyl and a hydroxyl functional group (-CO.OH); characteristic of carboxylic acids.
Fats have important functions, and many vitamins are fat-soluble. Fats serve as a long-term storage form of fatty acids and act as a source of energy. They also provide insulation for the body.
Glycerol and Fatty Acids
A fat molecule consists of two main components: glycerol and fatty acids. Glycerol is an alcohol with three carbons, five hydrogens, and three hydroxyls (OH) groups. Fatty acids have a long chain of hydrocarbons with a carboxyl group attached and may have 4-36 carbons; however, most of them have 12-18. In a fat molecule, the fatty acids are attached to each of the three carbons of the glycerol molecule with an ester bond through the oxygen atom. During the ester bond formation, three molecules are released. Since fats consist of three fatty acids and glycerol, they are also called triacylglycerols or triglycerides.

Triacylglycerols: Triacylglycerol is formed by the joining of three fatty acids to a glycerol backbone in a dehydration reaction. Three molecules of water are released in the process.
Saturated vs. Unsaturated Fatty Acids
Fatty acids may be saturated or unsaturated. In a fatty acid chain, if there are only single bonds between neighboring carbons in the hydrocarbon chain, the fatty acid is said to be saturated. Saturated fatty acids are saturated with hydrogen since single bonds increase the number of hydrogens on each carbon. Stearic acid and palmitic acid, which are commonly found in meat, are examples of saturated fats.
When the hydrocarbon chain contains a double bond, the fatty acid is said to be unsaturated. Oleic acid is an example of an unsaturated fatty acid. Most unsaturated fats are liquid at room temperature and are called oils. If there is only one double bond in the molecule, then it is known as a monounsaturated fat; e.g. olive oil. If there is more than one double bond, then it is known as a polyunsaturated fat; e.g. canola oil. Unsaturated fats help to lower blood cholesterol levels whereas saturated fats contribute to plaque formation in the arteries.
Unsaturated fats or oils are usually of plant origin and contain cis unsaturated fatty acids. Cis and trans indicate the configuration of the molecule around the double bond. If hydrogens are present in the same plane, it is referred to as a cis fat; if the hydrogen atoms are on two different planes, it is referred to as a trans fat. The cis double bond causes a bend or a “kink” that prevents the fatty acids from packing tightly, keeping them liquid at room temperature.
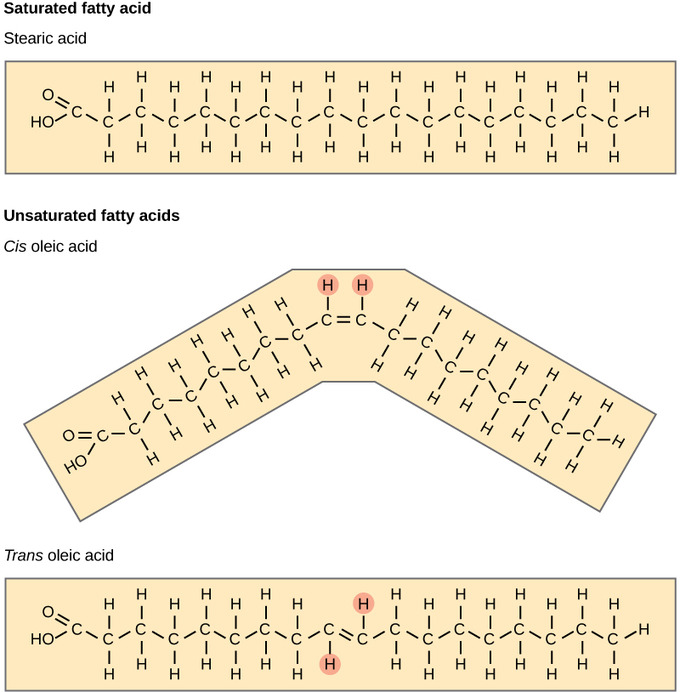
Fatty Acids: Saturated fatty acids have hydrocarbon chains connected by single bonds only. Unsaturated fatty acids have one or more double bonds. Each double bond may be in a cis or trans configuration. In the cis configuration, both hydrogens are on the same side of the hydrocarbon chain. In the trans configuration, the hydrogens are on opposite sides. A cis double bond causes a kink in the chain.
Trans Fats
In the food industry, oils are artificially hydrogenated to make them semi-solid and of a consistency desirable for many processed food products. During this hydrogenation process, gas is bubbled through oils to solidify them, and the double bonds of the cis-conformation in the hydrocarbon chain may be converted to double bonds in the trans-conformation.
Margarine, some types of peanut butter, and shortening are examples of artificially hydrogenated trans fats. Recent studies have shown that an increase in trans fats in the human diet may lead to an increase in levels of low-density lipoproteins (LDL), or “bad” cholesterol, which in turn may lead to plaque deposition in the arteries, resulting in heart disease. Many fast-food restaurants have recently banned the use of trans fats, and food labels are required to display the trans fat content.
Essential Fatty Acids

Omega Fatty Acids: Alpha-linolenic acid is an example of an omega-3 fatty acid. It has three cis double bonds and, as a result, a curved shape. For clarity, the carbons are not shown. Each singly bonded carbon has two hydrogens associated with it, also not shown.
Essential fatty acids are fatty acids required for biological processes, but not synthesized by the human body. Consequently, they have to be supplemented through ingestion via the diet and are nutritionally very important. Omega-3 fatty acid, or alpha-linoleic acid (ALA), falls into this category and is one of only two fatty acids known to be essential for humans (the other being omega-6 fatty acid, or linoleic acid). These polyunsaturated fatty acids are called omega-3 because the third carbon from the end of the hydrocarbon chain is connected to its neighboring carbon by a double bond. Salmon, trout, and tuna are good sources of omega-3 fatty acids.
Research indicates that omega-3 fatty acids reduce the risk of sudden death from heart attacks, reduce triglycerides in the blood, lower blood pressure, and prevent thrombosis by inhibiting blood clotting. They also reduce inflammation and may help reduce the risk of some cancers in animals.
Phospholipids
Phospholipids are amphipathic molecules that make up the bilayer of the plasma membrane and keep the membrane fluid.
Key Points
Phospholipids consist of a glycerol molecule, two fatty acids, and a phosphate group that is modified by alcohol.
The phosphate group is the negatively charged polar head, which is hydrophilic.
The fatty acid chains are the uncharged, nonpolar tails, which are hydrophobic.
Since the tails are hydrophobic, they face the inside, away from the water, and meet in the inner region of the membrane.
Since the heads are hydrophilic, they face outward and are attracted to the intracellular and extracellular fluid.
If phospholipids are placed in water, they form into micelles, which are lipid molecules that arrange themselves in a spherical form in aqueous solutions.
Key Terms
- micelle: Lipid molecules that arrange themselves in a spherical form in aqueous solutions.
- amphipathic: Describing a molecule, such as a detergent, which has both hydrophobic and hydrophilic groups.
Defining Characteristics of Phospholipids

Phospholipid Molecule: A phospholipid is a molecule with two fatty acids and a modified phosphate group attached to a glycerol backbone. The phosphate may be modified by the addition of charged or polar chemical groups. Two chemical groups that may modify the phosphate, choline, and serine, are shown here. Both choline and serine attach to the phosphate group at the position labeled R via the hydroxyl group indicated in green.
Phospholipids are major components of the plasma membrane, the outermost layer of animal cells. Like fats, they are composed of fatty acid chains attached to a glycerol backbone. Unlike triglycerides, which have three fatty acids, phospholipids have two fatty acids that help form a diacylglycerol. The third carbon of the glycerol backbone is also occupied by a modified phosphate group. However, just a phosphate group attached to a diacylglycerol does not qualify as a phospholipid. This would be considered a phosphatidate (diacylglycerol 3-phosphate), the precursor to phospholipids. To qualify as a phospholipid, the phosphate group should be modified by alcohol. Phosphatidylcholine and phosphatidylserine are examples of two important phospholipids that are found in plasma membranes.
Structure of a Phospholipid Molecule
A phospholipid is an amphipathic molecule which means it has both a hydrophobic and a hydrophilic component. A single phospholipid molecule has a phosphate group on one end, called the “head,” and two side-by-side chains of fatty acids that make up the lipid “tails.” The phosphate group is negatively charged, making the head polar and hydrophilic, or “water-loving.” The phosphate heads are thus attracted to the water molecules in their environment.
The lipid tails, on the other hand, are uncharged, nonpolar, and hydrophobic, or “water-fearing.” A hydrophobic molecule repels and is repelled by water. Some lipid tails consist of saturated fatty acids and some contain unsaturated fatty acids. This combination adds to the fluidity of the tails that are constantly in motion.
Phospholipids and Biological Membranes

Phospholipid Bilayer: The phospholipid bilayer consists of two adjacent sheets of phospholipids, arranged tail to tail. The hydrophobic tails associate with one another, forming the interior of the membrane. The polar heads contact the fluid inside and outside of the cell.
The cell membrane consists of two adjacent layers of phospholipids, which form a bilayer. The fatty acid tails of phospholipids face inside, away from water, whereas the phosphate heads face the outward aqueous side. Since the heads face outward, one layer is exposed to the interior of the cell and one layer is exposed to the exterior. As the phosphate groups are polar and hydrophilic, they are attracted to water in the intracellular fluid.
Because of the phospholipids chemical and physical characteristics, the lipid bilayer acts as a semipermeable membrane; only lipophilic solutes can easily pass the phospholipid bilayer. As a result, there are two distinct aqueous compartments on each side of the membrane. This separation is essential for many biological functions, including cell communication and metabolism.
Membrane Fluidity

Micelles: An example of micelles in water.
A cell’s plasma membrane contains proteins and other lipids (such as cholesterol) within the phospholipid bilayer. Biological membranes remain fluid because of the unsaturated hydrophobic tails, which prevent phospholipid molecules from packing together and forming a solid.
If a drop of phospholipids is placed in water, the phospholipids spontaneously form a structure known as a micelle, with their hydrophilic heads oriented toward the water. Micelles are lipid molecules that arrange themselves in a spherical form in an aqueous solution. The formation of a micelle is a response to the amphipathic nature of fatty acids, meaning that they contain both hydrophilic and hydrophobic regions.
Steroids
Steroids, like cholesterol, play roles in reproduction, absorption, metabolism regulation, and brain activity.
Key Points
Steroids are lipids because they are hydrophobic and insoluble in water, but they do not resemble lipids since they have a structure composed of four fused rings.
Cholesterol is the most common steroid and is the precursor to vitamin D, testosterone, estrogen, progesterone, aldosterone, cortisol, and bile salts.
Cholesterol is a component of the phospholipid bilayer and plays a role in the structure and function of membranes.
Steroids are found in the brain and alter electrical activity in the brain.
Because they can tone down receptors that communicate messages from neurotransmitters, steroids are often used in anesthetic medicines.
Key Terms
- neurotransmitter: any substance, such as acetylcholine or dopamine, responsible for sending nerve signals across a synapse between two neurons
- osmoregulation: the homeostatic regulation of osmotic pressure in the body in order to maintain a constant water content
- hormone: any substance produced by one tissue and conveyed by the bloodstream to another to affect physiological activity
Structure of Steroid Molecules
Unlike phospholipids and fats, steroids have a fused ring structure. Although they do not resemble the other lipids, they are grouped with them because they are also hydrophobic and insoluble in water. All steroids have four linked carbon rings, and many of them, like cholesterol, have a short tail. Many steroids also have the –OH functional group, and these steroids are classified as alcohols called sterols.

Steroid Structures: Steroids, such as cholesterol and cortisol, are composed of four fused hydrocarbon rings.
Cholesterol
Cholesterol is the most common steroid and is mainly synthesized in the liver; it is the precursor to vitamin D. Cholesterol is also a precursor to many important steroid hormones like estrogen, testosterone, and progesterone, which are secreted by the gonads and endocrine glands. Therefore, steroids play very important roles in the body’s reproductive system. Cholesterol also plays a role in synthesizing the steroid hormones aldosterone, which is used for osmoregulation, and cortisol, which plays a role in metabolism.
Cholesterol is also the precursor to bile salts, which help in the emulsification of fats and their absorption by cells. It is a component of the plasma membrane of animal cells and the phospholipid bilayer. Being the outermost structure in animal cells, the plasma membrane is responsible for the transport of materials and cellular recognition; and it is involved in cell-to-cell communication. Thus, steroids also play an important role in the structure and function of membranes.
It has also been discovered that steroids can be active in the brain where they affect the nervous system, These neurosteroids alter electrical activity in the brain. They can either activate or tone down receptors that communicate messages from neurotransmitters. Since these neurosteroids can tone down receptors and decrease brain activity, steroids are often used in anesthetic medicines.
References

